Abstract
Purpose of Review
Despite effective available treatments, gout management is often unsuccessful in getting patients to target serum urate goal and in managing flares in the setting of comorbidities. Studies addressing future treatment options for short- and long-term management are reviewed.
Recent Findings
URAT-1 blocking agents have been helpful but have had limitations related to effects on renal function, lack of efficacy with renal impairment, and potential to increase renal stones. Dotinurad may function in the setting of decreased renal function. Arhalofenate has anti-URAT-1 activity and may also blunt gout flares. A new xanthine oxidase inhibitor (XOI), tigulixostat, is under study. New uricase treatments manufactured in combination with agents that can reduce immunogenicity may make uricase treatment simpler. A unique strategy of inhibiting gut uricase may offer the benefits of avoiding systemic absorption. For gout flares, IL-1β inhibitor studies in progress include different dosing schedules. Dapansutrile, an oral agent under investigation, inhibits activation of the NLRP3 inflammasome and may be an effective anti-inflammatory.
Summary
New treatments for gout that are under study may work in the setting of comorbidities, simplify management, utilize new mechanisms, or have reduced side effects.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: A systematic review and meta-analysis based on observational cohort studies. BMC Nephrology. 2014;15:122. https://doi.org/10.1186/1471-2369-15-122.
Cicero AF, Salvi P, D’Addato S, Rosticci M, Borghi C. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J Hyper. 2014;32(1):57–64. https://doi.org/10.1097/HJH.0b013e328365b916.
Choi HK, McCormick N, Yokose C. Excess comorbidities in gout: the causal paradigm and pleiotropic approaches to care. Nature Rev Rheuma. 2022;18(2):97–111. https://doi.org/10.1038/s41584-021-00725-9.
Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PloS One. 2013;8(2):e56864. https://doi.org/10.1371/journal.pone.0056864.
Cipolletta E, Tata LJ, Nakafero G, Avery AJ, Mamas MA, Abhishek A. Risk of venous thromboembolism with gout flares. Arthritis & rheumatology (Hoboken, NJ). 2023. https://doi.org/10.1002/art.42480.
Edwards NL, Sundy JS, Forsythe A, Blume S, Pan F, Becker MA. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Eco. 2011;14(1):10–5. https://doi.org/10.3111/13696998.2010.540874.
Flores NM, Nuevo J, Klein AB, Baumgartner S, Morlock R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Eco. 2019;22(1):1–6. https://doi.org/10.1080/13696998.2018.1532904.
Blake KEG, Saag JL, Saag KG. What’s new on the front-line of gout pharmacotherapy? Exp Opinion Pharma. 2022;23(4):453–64. https://doi.org/10.1080/14656566.2021.2020249.
Saag KG, Fitz-Patrick D, Kopicko J, Fung M, Bhakta N, Adler S, et al. Lesinurad Combined with allopurinol: a randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study). Arthritis Rheuma (Hoboken, NJ). 2017;69(1):203–12. https://doi.org/10.1002/art.39840.
Bardin T, Keenan RT, Khanna PP, Kopicko J, Fung M, Bhakta N, et al. Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Annals Rheumatic Dis. 2017;76(5):811–20. https://doi.org/10.1136/annrheumdis-2016-209213.
Hosoya T, Sano T, Sasaki T, Fushimi M, Ohashi T. Clinical efficacy and safety of dotinurad, a novel selective urate reabsorption inhibitor, in Japanese hyperuricemic patients with or without gout: randomized, multicenter, double-blind, placebo-controlled, parallel-group, confirmatory phase 2 study. Clin Exp Nephrology. 2020;24(Suppl 1):53–61. https://doi.org/10.1007/s10157-019-01818-2.
Hosoya T, Sano T, Sasaki T, Fushimi M, Ohashi T. Dotinurad versus benzbromarone in Japanese hyperuricemic patient with or without gout: a randomized, double-blind, parallel-group, phase 3 study. Clinical and experimental nephrology. 2020;24(Suppl 1):62–70. https://doi.org/10.1007/s10157-020-01849-0.
Hosoya T, Furuno K, Kanda S. A non-inferiority study of the novel selective urate reabsorption inhibitor dotinurad versus febuxostat in hyperuricemic patients with or without gout. Clinical and experimental nephrology. 2020;24(Suppl 1):71–9. https://doi.org/10.1007/s10157-020-01851-6.Arandomizeddouble-blindedstudycomparingdotinuradtofebuxostatonceagainshowednon-inferiorityintheabilitytoloweruricacid.
Hosoya T, Fushimi M, Okui D, Sasaki T, Ohashi T. Open-label study of long-term administration of dotinurad in Japanese hyperuricemic patients with or without gout. Clinical and experimental nephrology. 2020;24(Suppl 1):80–91. https://doi.org/10.1007/s10157-019-01831-5.
Yanai H, Katsuyama H, Hakoshima M, Adachi H. Urate transporter 1 can be a therapeutic target molecule for chronic kidney disease and diabetic kidney disease: a retrospective longitudinal study. Biomedicines. 2023;11(2). https://doi.org/10.3390/biomedicines11020567.
Fitz-Patrick D, Roberson K, Niwa K, Fujimura T, Mori K, Hall J, et al. Safety and efficacy of verinurad, a selective URAT1 inhibitor, for the treatment of patients with gout and/or asymptomatic hyperuricemia in the United States and Japan: findings from two phase II trials. Modern rheumatology. 2019;29(6):1042–52. https://doi.org/10.1080/14397595.2018.1538003.
Stack AG, Dronamraju N, Parkinson J, Johansson S, Johnsson E, Erlandsson F, et al. Effect of intensive urate lowering with combined verinurad and febuxostat on albuminuria in patients with type 2 diabetes: a randomized trial. Ame J Kidney Diseases: official J National Kidney Foundation. 2021;77(4):481–9. https://doi.org/10.1053/j.ajkd.2020.09.009.
Heerspink HJL, Stack AG, Terkeltaub R, Greene TA, Inker LA, Bjursell M, et al. Rationale, design, demographics and baseline characteristics of the randomized, controlled, phase 2b SAPPHIRE study of verinurad plus allopurinol in patients with chronic kidney disease and hyperuricaemia. Nephrology, Dialysis, Transplantation: official Publ Eur Dialysis Transplant Assoc - European Renal Assoc. 2022;37(8):1461–71. https://doi.org/10.1093/ndt/gfab237.
McWherter C, Choi YJ, Serrano RL, Mahata SK, Terkeltaub R, Liu-Bryan R. Arhalofenate acid inhibits monosodium urate crystal-induced inflammatory responses through activation of AMP-activated protein kinase (AMPK) signaling. Arthritis Res Therapy. 2018;20(1):204. https://doi.org/10.1186/s13075-018-1699-4.
• Poiley J, Steinberg AS, Choi YJ, Davis CS, Martin RL, McWherter CA, et al. A randomized, double-blind, active- and placebo-controlled efficacy and safety study of arhalofenate for reducing flare in patients with gout. Arthritis & rheumatology (Hoboken, NJ). 2016;68(8):2027-34. https://doi.org/10.1002/art.39684. Arhalofenate 12-week phase IIb double-blinded RCT comparing arhalofenate to allopurinol and colchicine finding a modest reduction in uric acid and incident flare rates without renal issues.
3.Terkeltaub R LJ, Min J, Shin S, Saag KG. Serum urate-lowering efficacy and safety of tigulixostat in gout patients with hyperuricemia: a randomized, double-blind, placebo-controlled, dose-finding trial (CLUE). Arthritis & Rheumatology. 2023. https://doi.org/10.1002/art.42447. Latest data on tigulixostat findings demonstrated the capability of moderate to high-dose drug to reach a serum urate goal early in the trial and maintain control.
ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT05586958. Accessed 19 Apr 2023.
ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT05586971. Accessed 19 Apr 2023.
Padda IS BR, Parmar M. Pegloticase. In: StatPearls. 2023 January ed. Treasure Island (FL): StatPearls Publishing. 2023. https://www.ncbi.nlm.nih.gov/books/NBK572054/Pegloticase. Accessed 19 Apr 2023.
Schlesinger NLP. Pegloticase treatment of chronic refractory gout: update on efficacy and safety. Seminars Arthritis Rheumatism. 2020;50(3S):S31–8. https://doi.org/10.1016/j.semarthrit.2020.04.011.
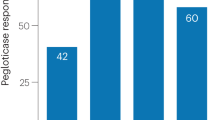
Albert JHT, LaMoreaux B. Increased efficacy and tolerability of pegloticase in patients with uncontrolled gout co-treated with methotrexate: a retrospective study. Rheumatol Therapy. 2020;7(3):639–48. https://doi.org/10.1007/s40744-020-00222-7.
Botson JKPJ. Pretreatment and coadministration with methotrexate improved durability of pegloticase response: an observational, proof-of-concept case series. Journal of Clinical Rheumatology. 2022;28(1):e129–34. https://doi.org/10.1097/RHU.0000000000001639.
Botson JK OK, LaMoreaux B, Zhao L, Weinblatt ME, Peterson J. Improved joint and patient-reported health assessments with pegloticase plus methotrexate co-therapy in patients with uncontrolled gout: 12-month exploratory outcomes of the MIRROR open-label trial. Arthritis research & therapy. 2022;24(1). https://doi.org/10.1186/s13075-022-02979-4.
Sands EKA, Johnston L, Kishimoto TK. THU0422 SEL-212: enhanced serum uric acid control in hyperuricemic patients through selective mitigation of anti-drug antibodies against pegsiticase. Annals of the rheumatic diseases. 2017;76 367.
TK K. Development of ImmTOR tolerogenic nanoparticles for the mitigation of anti-drug antibodies. Frontiers in Immunology. 2020;11. https://doi.org/10.3389/fimmu.2020.00969.
ClinicalTrials.gov. 2016. https://clinicaltrials.gov/ct2/show/NCT02648269. Accessed 2016 January 7.
ClinicalTrials.gov. 2016. https://clinicaltrials.gov/ct2/show/NCT02959918. Accessed 2016 November 9.
Sands EKA, Johnston L, DeHaan W, Kishimoto TK. FRI0234 SEL-212: Selective mitigation of anti-drug antibodies against pegsiticase to control serum uric acid in hyperuricemic subjects. Annals Rheumatic Dis. 2018;77:658. https://doi.org/10.1136/annrheumdis-2018-eular.7396.
Smolinski S KA, Dehaan W, Johnston L, Azeem R, Kishimoto TK. SAT0402 SEL-212 Phase 2 clinical study in symptomatic gout patients: ImmTOR tolerogenic nanoparticles combined with pegadricase mitigates immunogenicity and enables sustained reduction of serum uric acid levels, low rate of gout flares and monthly dosing. Annals of the rheumatic diseases. 2019:1288-9. https://doi.org/10.1136/annrheumdis-2019-eular.7769.
ClinicalTrials.gov. 2019. https://clinicaltrials.gov/ct2/show/NCT03905512. Accessed 2019 April 5.
ClinicalTrials.gov. 2020 https://clinicaltrials.gov/ct2/show/NCT04596540. Accessed 19 Apr 2023.
Pierzynowska K DA, Mosiichuk N, Terkeltaub R, Szczurek P, Salido E, Pierzynowski S, Grujic D. Oral treatment with an engineered uricase, ALLN-346, reduces hyperuricemia, and uricosuria in urate oxidase-deficient mice. Frontiers in Medicine. 2020;7. https://doi.org/10.3389/fmed.2020.569215.
Clark DGD, Tosone C, Dahl N, Terkeltaub R. Phase 1 trials of novel oral enzyme therapy (ALLN-346) for hyperuricemia & gout: safety, pharmacodynamics, and lack of systemic absorption of single and multiple ascending doses in healthy volunteers. Annals of the rheumatic diseases. 2022;81:906–7. https://doi.org/10.1136/annrheumdis-2022-eular.843.
Terkeltaub RCD, Tosone C, Kandinov B, Zhang P, Dahl N, Grujic D, Goldfarb D. POS1157 Safety and efficacy of ALLN-346 oral enzyme therapy in patients with hyperuricemia and chronic kidney disease (CKD): results of the phase 2A study 201. Annals Rheumatic Dis. 2022;81:907. https://doi.org/10.1136/annrheumdis-2022-eular.1662.
Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–18. https://doi.org/10.1016/j.immuni.2013.11.010.
Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. https://doi.org/10.1038/nature04516.
Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Annals of the rheumatic diseases. 2012;71(11):1839–48. https://doi.org/10.1136/annrheumdis-2011-200908.
Janssen CA, Oude Voshaar MAH, Vonkeman HE, Jansen T, Janssen M, Kok MR, et al. Anakinra for the treatment of acute gout flares: a randomized, double-blind, placebo-controlled, active-comparator, non-inferiority trial. Rheumatology (Oxford). 2019. https://doi.org/10.1093/rheumatology/key402. This is a double-blind, double-dummy, non-inferiority RCT comparing anakinra to naproxen, colchicine, or prednisone for management of acute gout flare demonstrating non-inferiority of anakinra to usual care.
Saag KG, Khanna PP, Keenan RT, Ohlman S, Osterling Koskinen L, Sparve E, et al. A randomized, phase II study evaluating the efficacy and safety of anakinra in the treatment of gout flares. Arthritis Rheuma (Hoboken, NJ). 2021;73(8):1533–42. https://doi.org/10.1002/art.41699. This double-blind, double-dummy superiority RCT comparing anakinra to triamcinolone for the management of acute gout flare found no statistically significant difference between study arms in pain reduction or time to resolution of pain.
van de Laar CJ, Janssen CA, Janssen M, Oude Voshaar MAH, Al MJ, van de Laar M. Model-based cost-effectiveness analyses comparing combinations of urate lowering therapy and anti-inflammatory treatment in gout patients. PloS one. 2022;17(1):e0261940. https://doi.org/10.1371/journal.pone.0261940.
Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Annals Rheumatic Dis. 2017;76(1):29–42. https://doi.org/10.1136/annrheumdis-2016-209707.
FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res. 2020;72(6):744–60. https://doi.org/10.1002/acr.24180.
Guttmann A, Krasnokutsky S, Pillinger MH, Berhanu A. Pegloticase in gout treatment-safety issues, latest evidence and clinical considerations. Therapeutic Adv Drug Safety. 2017;8(12):379–88. https://doi.org/10.1177/2042098617727714.
Assistance Publique - Hôpitaux de P: Anakinra vs prednisone to treat gout flare in patients with chronic kidney disease stage 4/5 or renal transplantation. https://ClinicalTrials.gov/show/NCT04844814 (2025). Accessed.
Twi Biotechnology I: a proof-of-concept study of AC-201 to prevent gout flares. https://ClinicalTrials.gov/show/NCT01712204 (2013). Accessed.
Pharm R, Covance, Laboratories ZAOU, Data Matrix S, Pharmaceutical Analytics Center LLC, Ltd OCTR: safety, tolerability, pharmacokinetics and pharmacodynamics evaluation of RPH-104 administered at different doses to patients with acute gout attack. https://ClinicalTrials.gov/show/NCT04067492 (2020). Accessed.
Alten R, Krüger K, Rellecke J, Schiffner-Rohe J, Behmer O, Schiffhorst G, et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adherence. 2016;10:2217–28. https://doi.org/10.2147/ppa.S117774.
Klück V, Jansen T, Janssen M, Comarniceanu A, Efdé M, Tengesdal IW, et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2020;2(5):e270-e80. https://doi.org/10.1016/s2665-9913(20)30065-5. This is an upcoming phase II/III randomized, double-blind, placebo-controlled safety and efficacy study of dapansutrile (OLT1177), an oral small molecule that selectively binds and inhibits the NLRP3 inflammasome and is expected to be completed in October 2023.
Therapeutics H. Krystexxa (pegloticase) [prescribing information].
Rainey HBH, Yeo A, Lipsky P. THU0410 Companion immunosuppression with azathioprine increases the frequency of persistent responsiveness to pegloticase in patients with chronic refractory gout. Annals of the rheumatic diseases. 2020;79:442–3. https://doi.org/10.1136/annrheumdis-2020-eular.4642.
Masri KRP-SL, Winterling K, LaMoreaux B. Effect of leflunomide on pegloticase response rate in patients with uncontrolled gout: a retrospective study. Rheuma Therapy. 2022;9(2):555–63. https://doi.org/10.1007/s40744-021-00421-w.
Khanna PPKD, Cutter G, Foster J, Melnick J, Jaafar S, Biggers S, Rahman AKMF, Kuo HC, Feese M, Kivitz A, King C, Shergy W, Kent J, Peloso PM, Danila MI, Saag KG. Reducing immunogenicity of pegloticase with concomitant use of mycophenolate mofetil in patients with refractory gout: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis & Rheumatology. 2021;73(8):1523–32. https://doi.org/10.1002/art.41731.
Klück V CG, Mies L, Bukkems F, van Emst L, Bakker R, van Caam A; HINT consortium; Crişan TO, Joosten LAB. TGF-β is elevated in hyperuricemic individuals and mediates urate-induced hyperinflammatory phenotype in human mononuclear cells. Arthritis research & therapy. 2023;25(1). https://doi.org/10.1186/s13075-023-03001-1.
Ma T, Liu X, Cen Z, Xin C, Guo M, Zou C, et al. MicroRNA-302b negatively regulates IL-1β production in response to MSU crystals by targeting IRAK4 and EphA2. Arthritis research & therapy. 2018;20(1):34. https://doi.org/10.1186/s13075-018-1528-9.
Mirzaesmaeili A, Zangiabadi S, Raspanti J, Akram A, Inman RD, Abdul-Sater AA. Cutting edge: negative regulation of inflammasome activation by TRAF1 can limit gout. J Immunol. 2023. https://doi.org/10.4049/jimmunol.2200465.
Elsaid K, Merriman TR, Rossitto LA, Liu-Bryan R, Karsh J, Phipps-Green A, et al. Amplification of inflammation by lubricin deficiency implicated in incident, erosive gout independent of hyperuricemia. Arthritis & rheumatology (Hoboken, NJ). 2022. https://doi.org/10.1002/art.42413.
Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–43. https://doi.org/10.1592/phco.28.4.437.
Harrold LR, Andrade SE. Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Seminars in arthritis and rheumatism. 2009;38(5):396–402. https://doi.org/10.1016/j.semarthrit.2008.01.011.
Doherty M, Jenkins W, Richardson H, Sarmanova A, Abhishek A, Ashton D, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet (London, England). 2018;392(10156):1403–12. https://doi.org/10.1016/s0140-6736(18)32158-5.
Fields TR, Batterman A. How can we improve disease education in people with gout? Current Rheumatol Rep. 2018;20(3):12. https://doi.org/10.1007/s11926-018-0720-x.
Fields JK, Günther S, Sundberg EJ. Structural basis of IL-1 family cytokine signaling. Front Immunol. 2019;10:1412. https://doi.org/10.3389/fimmu.2019.01412.
Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22(4):189–95. https://doi.org/10.1016/j.cytogfr.2011.10.001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
TF disclosures are as follows: Horizon Pharmaceuticals (payment for consulting on gout exhibit), Avion Pharmaceuticals (Advisory Board), and Novartis Pharmaceuticals (payment for consultation). Authors KY, LY, and GB declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Summary Points
• Current and future investigations into oral formulations and more proximal inflammasome pathway modulation for acute gout flares are of great research interest, as are further studies into IL-1β blocking agents.
• The combination uricase/immunosuppressive SEL-212 avoids the need for co-administration with methotrexate or other immunosuppressants to reduce the formation of neutralizing ADAs and maintain urate-lowering efficacy. Monthly infusion dosing is another potential advantage.
• Bioengineered uricase ALLN-346 is positioned to take advantage of the intestinal uric acid excretion pathway and may prove especially useful in patients with CKD. Oral administration is an advantage, and early studies showed a lack of adverse reactions.
• Tigulixostat is a selective xanthine oxidase inhibitor with effective sUA lowering capabilities at even moderate dose which may be easier to titrate than existing agents.
• The URAT1 agent dotinurad has promise as a monotherapy, possibly without significant renal problems, and could be effective even in mild CKD.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yip, K., Braverman, G., Yue, L. et al. Pipeline Therapies for Gout. Curr Rheumatol Rep 26, 69–80 (2024). https://doi.org/10.1007/s11926-023-01128-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-023-01128-3