Abstract
Purpose of Review
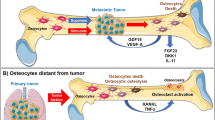
The formation of a pre-metastatic niche (PMN), in which primary cancer cells prime the distant site to be favorable to their engraftment and survival, may help explain the strong osteotropism observed in multiple cancers, such as breast and prostate. PMN formation, which includes extracellular matrix remodeling, increased angiogenesis and vascular permeability, enhanced bone marrow-derived cell recruitment and immune suppression, has mostly been described in soft tissues. In this review, we summarize current literature of PMN formation in bone. We also present evidence of a potential role for osteocytes to be the primary mediators of PMN development.
Recent Findings
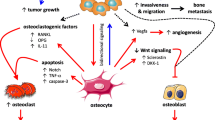
Osteocytes regulate the bone microenvironment in myriad ways beyond canonical bone tissue remodeling, including changes that contribute to PMN formation. Perilacunar tissue remodeling, which has been observed in both bone and non-bone metastatic cancers, is a potential mechanism by which osteocyte-cancer cell signaling stimulates changes to the bone microenvironment. Osteocytes also protect against endothelial permeability, including that induced by cancer cells, in a loading-mediated process. Finally, osteocytes are potent regulators of cells within the bone marrow, including progenitors and immune cells, and might be involved in this aspect of PMN formation.
Summary
Osteocytes should be examined for their role in PMN formation.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jiang W, Rixiati Y, Zhao B, Li Y, Tang C, Liu J. Incidence, prevalence, and outcomes of systemic malignancy with bone metastases. https://doi.org/10.1177/2309499020915989.
Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2014;17(6):816–26. https://doi.org/10.1038/ncb3169.
Zeng Z, et al. Cancer-derived exosomal miR-25–3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1). https://doi.org/10.1038/S41467-018-07810-W.
Paolillo M, Schinelli S. Extracellular matrix alterations in metastatic processes. Int J Mol Sci. 2019;20(19). https://doi.org/10.3390/IJMS20194947.
So A, Chin J, Fleshner N, Saad F. Management of skeletal-related events in patients with advanced prostate cancer and bone metastases: incorporating new agents into clinical practice. Can Urol Assoc J. 2012;6(6):465. https://doi.org/10.5489/CUAJ.12149.
Mayhew V, Omokehinde T, Johnson RW. Tumor dormancy in bone. 2019. https://doi.org/10.1002/cnr2.1156.
Svensson E, Christiansen CF, Ulrichsen SP, Rørth MR, Sørensen HT. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7(9). https://doi.org/10.1136/BMJOPEN-2017-016022.
Fröbel J, et al. The hematopoietic bone marrow niche ecosystem. Front Cell Dev Biol. 2021;9:1958. https://doi.org/10.3389/FCELL.2021.705410/BIBTEX.
Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, Mccauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone 1. CANCER Res. 2002;62:1832–1837. Accessed: Apr. 20, 2023. [Online]. Available: http://aacrjournals.org/cancerres/article-pdf/62/6/1832/2501673/1832.pdf.
Müller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. https://doi.org/10.1038/35065016.
David Roodman G. Mechanisms of bone metastasis. N Engl J Med. 2004:350:1655–64. Accessed: Apr. 20, 2023. [Online]. Available: https://www.nejm.org.
Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001;1(5):621–32. https://doi.org/10.2174/1566524013363339.
Wang H, et al. The Osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27(2):193–210. https://doi.org/10.1016/J.CCELL.2014.11.017.
Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22(5):559–74. https://doi.org/10.1101/GAD.1636908.
Dong Q, Liu X, Cheng K, Sheng J, Kong J, Liu T. Pre-metastatic niche formation in different organs induced by tumor extracellular vesicles. Front Cell Dev Biol. 2021;9. https://doi.org/10.3389/FCELL.2021.733627.
• Hsu YL, et al. Bone-marrow-derived cell-released extracellular vesicle miR-92a regulates hepatic pre-metastatic niche in lung cancer. Oncogene. 2020;39(4):739–53. https://doi.org/10.1038/S41388-019-1024-Y. Activation of hepatic stellate cells, which play a similar in liver as osteocytes in bone, results in extracellular matrix remodeling as part of pre-metastatic niche formation.
• Yuan X, et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics. 2021;11(3):1429–45. https://doi.org/10.7150/thno.45351. Breast cancer-derived exosomes travel to and affect osteoclast activity to initiate pre-metastatic niche formation.
Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94(1):5. https://doi.org/10.1007/S00223-013-9790-Y.
Florencio-Silva R, Rodrigues Da G, Sasso S, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. 2015. https://doi.org/10.1155/2015/421746.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. https://doi.org/10.1002/JBMR.320.
Galea GL, Lanyon LE, Price JS. Sclerostin’s role in bone’s adaptive response to mechanical loading. 2016. https://doi.org/10.1016/j.bone.2016.10.008.
Tsourdi E, Jähn K, Rauner M, Busse B, Bonewald LF. Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact. 2018;18(3):292. Accessed: Apr. 20, 2023. [Online]. Available: /pmc/articles/PMC6146198/.
Jähn-Rickert K, Zimmermann EA. Potential role of perilacunar remodeling in the progression of osteoporosis and implications on age-related decline in fracture resistance of bone. Curr Osteoporos Rep. 2021;19(4):391–402. https://doi.org/10.1007/S11914-021-00686-8.
Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30(5):668–81. https://doi.org/10.1016/J.CCELL.2016.09.011.
Qi Y, Zhao T, Li R, Han M. Macrophage-secreted S100A4 supports breast cancer metastasis by remodeling the extracellular matrix in the premetastatic niche. Biomed Res Int. 2022;2022. https://doi.org/10.1155/2022/9895504.
Medeiros B, et al. Triple-negative primary breast tumors induce supportive premetastatic changes in the extracellular matrix and soluble components of the lung microenvironment. Cancers (Basel). 2020;12(1). https://doi.org/10.3390/CANCERS12010172.
Martin TJ, Johnson RW. Multiple actions of parathyroid hormone-related protein in breast cancer bone metastasis. Br J Pharmacol. 2021;178(9):1923–35. https://doi.org/10.1111/BPH.14709.
Athonvarangkul D, Wysolmerski JJ. Crosstalk within a brain-breast-bone axis regulates mineral and skeletal metabolism during lactation. Front Physiol. 2023;14. https://doi.org/10.3389/FPHYS.2023.1121579.
Qing H, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018. https://doi.org/10.1002/JBMR.1567.
Kyvernitakis I, Rachner TD, Urbschat A, Hars O, Hofbauer LC, Hadji P. Effect of aromatase inhibition on serum levels of sclerostin and dickkopf-1, bone turnover markers and bone mineral density in women with breast cancer. J Cancer Res Clin Oncol. 2014;140(10):1671–80. https://doi.org/10.1007/S00432-014-1726-Z/TABLES/3.
• Hesse E, Schröder S, Brandt D, Pamperin J, Saito H, Taipaleenmäki H. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. 2019;5(9). https://doi.org/10.1172/JCI.INSIGHT.125543. Inhibition of sclerostin, primarily secreted by osteocytes, reduced breast cancer cell trafficking to bone, growth of cells that did engraft, and subsequent tumor-induced bone loss.
Sekita A, Matsugaki A, Ishimoto T, Nakano T. Synchronous disruption of anisotropic arrangement of the osteocyte network and collagen/apatite in melanoma bone metastasis. J Struct Biol. 2017;197(3):260–70. https://doi.org/10.1016/J.JSB.2016.12.003.
•• Pin F, et al. Non-bone metastatic cancers promote osteocyte-induced bone destruction. Cancer Lett. 2021;520:80–90. https://doi.org/10.1016/J.CANLET.2021.06.030. Demonstrates that osteocytes and distant tumor cells signal to each other, with resulting aberrant osteocyte perilacunar remodeling.
Peinado H, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17. https://doi.org/10.1038/nrc.2017.6.
Zhou W, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15. https://doi.org/10.1016/J.CCR.2014.03.007.
Ma Z, et al. Tumor-derived exosomal miR-3157–3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Dis. 2021;12(9). https://doi.org/10.1038/S41419-021-04037-4.
• Prasadam I, Zhou Y, Du Z, Chen J, Crawford R, Xiao Y. Osteocyte-induced angiogenesis via VEGF-MAPK-dependent pathways in endothelial cells. https://doi.org/10.1007/s11010-013-1840-2. Osteocytes regulate angiogenesis through signaling to endothelial cells via VEGF.
Cheung WY, Liu C, Tonelli-Zasarsky RML, Simmons CA, You L. Osteocyte apoptosis is mechanically regulated and induces angiogenesis in vitro. J Orthop Res. 2011;29(4):523–30. https://doi.org/10.1002/JOR.21283.
Choy MHV, et al. How much do we know about the role of osteocytes in different phases of fracture healing? A systematic review. J Orthop Transl. 2019;21:111–21. https://doi.org/10.1016/J.JOT.2019.07.005.
Hadjiargyrou M, Ahrens W, Rubin CT. Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair. J Bone Miner Res. 2000;15(6):1014–23. https://doi.org/10.1359/JBMR.2000.15.6.1014.
Rocha CA, Cestari TM, Vidotti HA, De Assis GF, Garlet GP, Taga R. Sintered anorganic bone graft increases autocrine expression of VEGF, MMP-2 and MMP-9 during repair of critical-size bone defects. J Mol Histol. 2014;45(4):447–61. https://doi.org/10.1007/S10735-014-9565-4.
Bennett BJ, et al. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2006;26(9):2117–24. https://doi.org/10.1161/01.ATV.0000236428.91125.E6/FORMAT/EPUB.
Min J-K, et al. Receptor activator of nuclear factor (NF)-B ligand (RANKL) increases vascular permeability: impaired permeability and angiogenesis in eNOS-deficient mice. 2007. https://doi.org/10.1182/blood-2006-06-029298.
Ma YHV, et al. Mechanical regulation of breast cancer migration and apoptosis via direct and indirect osteocyte signaling. J Cell Biochem. 2018;119(7):5665–75. https://doi.org/10.1002/JCB.26745.
•• Ma YHV, Xu L, Mei X, Middleton K, You L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J Cell Biochem. 2019;120(5):7590–601. https://doi.org/10.1002/JCB.28034. Fluid flow-stimulated osteocytes reduced endothelial cell permeability, and breast cancer cell adhesion to and migration through endothelial layers. This result was mediated in part by reducing cancer cell secretion of proteases, such as MMP9.
McKenzie JA, Galbreath IM, Coello AF, Hixon KR, Silva MJ. VEGFA from osteoblasts is not required for lamellar bone formation following tibial loading. Bone. 2022;163. https://doi.org/10.1016/J.BONE.2022.116502.
Hadji P, et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1–12. https://doi.org/10.1016/J.JBO.2017.03.001.
Mosey H, et al. Sost deficiency does not alter bone’s lacunar or vascular porosity in mice. Front Mater. 2017;4:27. https://doi.org/10.3389/FMATS.2017.00027/BIBTEX.
McDonald MM, et al. Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectomized rats. J Orthop Res. 2012;30(10):1541–8. https://doi.org/10.1002/JOR.22109.
McDonald MM, et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017;129(26):3452–64. https://doi.org/10.1182/BLOOD-2017-03-773341.
• Zhao S, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13(1). https://doi.org/10.1186/S13045-020-00991-2. Colon cancer-secreted exosomes modulated cross-talk with the distant liver via macrophage polarization, of which osteocytes are also capable, and contributed formation of the pre-metastatic niche.
Zhong L, et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct Target Ther. 2021;61(1):1–16. https://doi.org/10.1038/s41392-020-00414-1.
Wang Y, Ding Y, Guo N, Wang S. MDSCs: key criminals of tumor pre-metastatic niche formation. Front Immunol. 2019;10(FEB):172. https://doi.org/10.3389/FIMMU.2019.00172/BIBTEX.
Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77(13):3655–65. https://doi.org/10.1158/0008-5472.CAN-16-3199.
Tie Y, Tang F, Wei Yq, Wei Xw. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol. 2022;15(1). https://doi.org/10.1186/S13045-022-01282-8.
•• Dwivedi A, Kiely PA, Hoey DA. Mechanically stimulated osteocytes promote the proliferation and migration of breast cancer cells via a potential CXCL1/2 mechanism. Biochem Biophys Res Commun. 2021;534:14–20. https://doi.org/10.1016/J.BBRC.2020.12.016. Fluid flow-stimulated osteocytes stimulated secretion of CXCL1 and CXCL12, cytokines that contribute to breast cancer homing to and engraftment in bone.
Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28(2):105–12. https://doi.org/10.1247/CSF.28.105.
Kordes C, Bock HH, Reichert D, May P, Häussinger D. Hepatic stellate cells: current state and open questions. Biol Chem. 2021;402(9):1021–32. https://doi.org/10.1515/HSZ-2021-0180.
Kang N. Mechanotransduction in liver diseases. Semin Liver Dis. 2020;40(1):84–90. https://doi.org/10.1055/S-0039-3399502/ID/JR1900042-23/BIB.
Sakamoto K, Schmidt JW, Wagner KU. Mouse models of breast cancer. Methods Mol Biol. 2015;1267:47–71. https://doi.org/10.1007/978-1-4939-2297-0_3.
Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006;8(4). https://doi.org/10.1186/BCR1530.
Main RP, Shefelbine SJ, Meakin LB, Silva MJ, van der Meulen MCH, Willie BM. Murine axial compression tibial loading model to study bone mechanobiology: implementing the model and reporting results. J Orthop Res. 2020;38(2):233–52. https://doi.org/10.1002/JOR.24466.
Falk CH, Liu B, Lynch ME. Bone mechanics in cancer. Encycl Bone Biol. 2020:445–457. https://doi.org/10.1016/B978-0-12-801238-3.11256-5.
Hu Y, et al. Bone/cartilage organoid on-chip: construction strategy and application. Bioact Mater. 2023;25:29–41. https://doi.org/10.1016/J.BIOACTMAT.2023.01.016.
Liu Y, Lin L, Qiao L. Recent developments in organ-on-a-chip technology for cardiovascular disease research. Anal Bioanal Chem. 2023. https://doi.org/10.1007/S00216-023-04596-9.
Ronaldson-Bouchard K, et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat Biomed Eng. 2022;6(4):351–71. https://doi.org/10.1038/S41551-022-00882-6.
Funding
This work was supported by the National Science Foundation (BMMB-2047187), the Cancer League of Colorado, Inc. (Grant #203445), and the Anschutz-Boulder Nexus Program (#9866).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest with the contents of this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Briggs, E.N., Lynch, M.E. The Role of Osteocytes in Pre-metastatic Niche Formation. Curr Osteoporos Rep 22, 105–114 (2024). https://doi.org/10.1007/s11914-023-00857-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-023-00857-9