Abstract
Purpose of Review
Obstructive sleep apnea (OSA), featured by chronic intermittent hypoxia (CIH), is an independent risk for systemic hypertension (HTN) and is associated with pulmonary hypertension (PH). The precise mechanisms underlying pulmonary vascular remodeling and PH in OSA are not fully understood. However, it has been suggested that lung tissue hypoxia, oxidative stress, and pro-inflammatory mediators following CIH exposure may contribute to PH.
Recent Findings
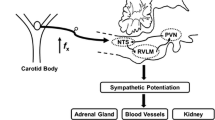
New evidences obtained in preclinical OSA models support that an enhanced carotid body (CB) chemosensory reactiveness to oxygen elicits sympathetic and renin-angiotensin system (RAS) overflow, which contributes to HTN. Moreover, the ablation of the CBs abolished the sympathetic hyperactivity and HTN in rodents exposed to CIH. Accordingly, it is plausible that the enhanced CB chemosensory reactivity may contribute to the pulmonary vascular remodeling and PH through the overactivation of the sympathetic-RAS axis. This hypothesis is supported by the facts that (i) CB stimulation increases pulmonary arterial pressure, (ii) denervation of sympathetic fibers in pulmonary arteries reduces pulmonary remodeling and pulmonary arterial hypertension (PAH) in humans, and (iii) administration of angiotensin-converting enzyme (ACE) or blockers of Ang II type 1 receptor (ATR1) ameliorates pulmonary remodeling and PH in animal models.
Summary
In this review, we will discuss the supporting evidence for a plausible contribution of the CB-induced sympathetic-RAS axis overflow on pulmonary vascular remodeling and PH induced by CIH, the main characteristic of OSA.

Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- AHI:
-
Apnea/hypopnea index
- Ang II:
-
Angiotensin II
- ATR1:
-
Angiotensin II receptor type 1
- ATR2:
-
Angiotensin II receptor type 2
- BP:
-
Arterial blood pressure
- BRS:
-
Baroreceptor reflex sensitivity
- CB:
-
Carotid body
- CIH:
-
Chronic intermittent hypoxia
- CPAP:
-
Continuous positive airway pressure
- CSN:
-
Carotid sinus nerve
- CVO:
-
Circumventricular organs
- ET-1:
-
Endothelin-1
- ETB:
-
Endothelin type B receptor
- HTN:
-
Systemic hypertension
- HIFs:
-
Hypoxia-induced factors
- IL-6:
-
Interleukin 6
- IL-1β:
-
Interleukin 1β
- ICAM:
-
Intercellular adhesion molecule 1
- MnSOD:
-
Manganese-dependent superoxide dismutase
- MCP-1:
-
Monocyte chemoattractant protein-1
- MSNA:
-
Muscle sympathetic nerve activity
- NA:
-
Nucleus ambiguous
- NF-kB:
-
Transcription nuclear factor κB
- NTS:
-
Nucleus of the tractus solitarious
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NOX2:
-
NADPH oxidase 2
- NOX4:
-
NADPH oxidase 4
- 3-NT:
-
3-Nitrotyrosine
- OSA:
-
Obstructive sleep apnea
- PASMC:
-
Pulmonary arterial smooth muscle cells
- PAH:
-
Pulmonary arterial hypertension
- PH:
-
Pulmonary hypertension
- PVN:
-
Paraventricular nucleus
- RAS:
-
Renin-angiotensin system
- ROS:
-
Reactive oxygen species
- RVSP:
-
Right ventricular systolic pressure
- RVH:
-
Right ventricular hypertrophy
- RVLM:
-
Rostral ventrolateral medulla
- SFO:
-
Subfornical organ
- SHR:
-
Spontaneous hypertensive rats
- TNF-α:
-
Tumor necrosis factor
- VCAM:
-
Vascular cell adhesion molecule
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16.
• Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. This is a comprehensive review of the pathophysiological consequences of sleep apnea.
Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33:1195–205.
Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea, oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177:369–75.
• Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease. Circulation. 2008;118:1080–111. A complete review of the cardiovascular consequences of OSA.
Gonçalves SC, Martinez D, Gus M, de Abreu-Silva EO, Bertoluci C, Dutra I, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest. 2007;132:1858–62.
Tonecny T, Tomas K, Virend K. Somers. Obstructive sleep apnea and hypertension an update. Hypertension. 2014;63:203–9.
Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8.
Fletcher EC, Lesske J, Behm R, Miller CC, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992a;72:1978–84.
Prabhakar NR, Kumar GK, Peng YJ. Sympatho-adrenal activation by chronic intermittent hypoxia. J Appl Physiol. 2012;113:1304–10.
Iturriaga R, Oyarce MP, Dias ACR. Role of carotid body in intermittent hypoxia-related hypertension. Curr Hyperten Rep. 2017;19:38.
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. Am Coll Cardiol. 2013;62:D34–41.
• Atwood CW Jr, McCrory D, Garcia JG, Abman SH, Ahearn GS, American College of Chest Physicians. Pulmonary artery hypertension and sleep-disordered breathing: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):72S–7S. A complete review of the relationship between pulmonary hypertension and sleep disorders.
Bosc LVG, Resta T, Walker B, Kanagy NL. Mechanisms of intermittent hypoxia induced hypertension. J Cell Mol Med. 2010;14:3–17.
Floras JS. Sleep apnea and cardiovascular disease: an enigmatic risk factor. Circ Res. 2018;122:1741–64.
Me AS, El-Desoky ME, Maaty AER, Abd-ElMaksoud AM, Suliman LA. Pulmonary hypertension in obstructive sleep apnea hypopnea syndrome. Egyp J Chest Dis Tuber. 2013;62:459–65.
Imran TF, Ghazipura M, Liu S, Hossain T, Ashtyani H, Kim B, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591–8.
Adegunsoye A, Ramachandran S. Etiopathogenetic mechanisms of pulmonary hypertension in sleep-related breathing disorders. Pulm Med. 2012;2012:273591.
Kholdani S, Fares WH, Mohsenin V. Pulmonary hypertension in obstructive sleep apnea: is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ. 2015;5:220–7.
Ismail K, Roberts K, Manning P, Manley C, Hill NS. OSA and pulmonary hypertension: time for a new look. Chest. 2015;147:847–61.
• Dunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LR, Mewburn JD, et al. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest. A complete review of pulmonary hypertension mechanisms. 2017;151:181–92.
Suresh K, Shimoda LA. Lung circulation. Compr Physiol. 2016;6:897–943.
•• Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92:367–520. A comprehensive review of hypoxic vasoconstriction in the lung.
Nara A, Nagai H, Shintani-Ishida K, Ogura S, Shimosawa T, Kuwahira I, et al. Pulmonary arterial hypertension in rats due to age-related arginase activation in intermittent hypoxia. Am J Respir Cell Mol Biol. 2015;53:184–92.
Jin H, Liu M, Zhang X, Pan J, Han J, Wang Y, et al. Grape seed procyanidin extract attenuates hypoxic pulmonary hypertension by inhibiting oxidative stress and pulmonary arterial smooth muscle cells proliferation. J Nutr Biochem. 2016;36:81–8.
Jin H, Wang Y, Zhou L, Liu L, Zhang P, Deng W, et al. Melatonin attenuates hypoxic pulmonary hypertension by inhibiting the inflammation and the proliferation of pulmonary arterial smooth muscle cells. J Pineal Res. 2014;57:442–50.
Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan THM, et al. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Moll Biol. 2009;40:601–9.
Cho HJ, Heo W, Han JW, Lee YH, Park JM, Kang MJ, Kim JY. Chronological change of right ventricle by chronic intermittent hypoxia in mice. Sleep. 2017;40.
Fagan KA. Selected Contribution: Pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol. 2001;90:2502–7.
Lu H, Wu X, Fu C, Zhou J, Li S. Lung injury and inflammation response by chronic intermittent hypoxia in rats. Sleep Sci Pract. 2017;1:1.
Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. New Eng J Med. 2004;351:1425–36.
Shao J, Wang P, Liu A, Du X, Bai J, Chen M. Punicalagin prevents hypoxic pulmonary hypertension via antioxidant effects in rats. Am J Chin Med. 2016;44:785–801.
Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–26.
Bakouboula B, Morel O, Faure A, Zobairi F, Jesel L, Trinh A, et al. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:536–43.
Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–9.
Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006:99675–91.
Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034.
Lu W, Kang J, Hu K, Tang S, Zhou X, Yu S, et al. Angiotensin-(1–7) inhibits inflammation and oxidative stress to relieve lung injury induced by chronic intermittent hypoxia in rats. Braz J Med Biol Res. 2016;49:e5431.
Xu XM, Yao D, Cai XD, Ding C, Lin QD, Wang LX, et al. Effect of chronic continual- and intermittent hypoxia-induced systemic inflammation on the cardiovascular system in rats. Sleep Breath. 2015;19:677–84.
Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–19.
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, et al. ROS and ROS-mediated cellular signaling. Oxidative Med Cell Longev. 2016;2016:4350965.
Zhang X, Rui L, Wang M, Lian H, Cai L. Sinomenine attenuates chronic intermittent hypoxia-induced lung injury by inhibiting inflammation and oxidative stress. Med Sci Monit. 2018;24:1574–80.
Yang CH, Zhuang WL, Shen YJ, Lai CJ, Kou YR. NADPH oxidase-derived ROS induced by chronic intermittent hypoxia mediates hypersensitivity of lung vagal C fibers in rats. Front Physiol. 2016;7:166.
Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. AJ Res Crit Care Med. 2011;183:152–6.
Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, et al. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–10.
Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, et al. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci U S A. 2012;109:1239–44.
Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–6.
• Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, et al. Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest. 2003;111:1519–27. Highlighted the role played by HIF-2α in pulmonary hypertension.
Cowburn AS, Crosby A, Macias D, Branco C, Colaço RD, Southwood M, et al. HIF2α-arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci U S A. 2016;113:8801–6.
Dai Z, Li M, Wharton J, Zhu M. Zhao YY.PHD2 deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through HIF-2α. Circulation. 2016;133:2447–58.
De Morais SDB, Shanks J, Zucker IH. Integrative physiological aspects of brain ras in hypertension. Curr Hypertense Rep. 2018;26(20):10.
Fung ML. The role of local renin-angiotensin system in arterial chemoreceptors in sleep-breathing disorders. Front Physiol. 2014;5:336.
Marshall RP. The pulmonary renin-angiotensin system. Curr Pharm Des. 2003;9:715–22.
Maron BA, Leopold JA. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension. Pulm Circ. 2013;4:200–10.
de Man FS, Tu L, Handoko ML, Rain S, Ruiter G, François C, et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:780–9.
Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, et al. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048–54.
• Morrel NW, Kenneth GM, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269:H1186–94. Pioneer work on the contribution of RAS on pulmonary hypertension.
Morrell NW, Upton PD, Higham MA, Yacoub MH, Polak JM, Wharton J. Angiotensin II stimulates proliferation of human pulmonary artery smooth muscle cells via the AT1 receptor. 1998;114:90S–91S.
Jia G, Aroor AR, Hill MA, Sowers JR. Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis. Hypertension. 2018;72:537–46.
Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98:1627–738.
Martyniuk TV, Chazova IE, Masenko VP, Volkov VN, Belenkov IN. Activity of renin-angiotensin-aldosterone system (RAAS) and vasopressin level in patients with primary pulmonary hypertension. Ter Arkh. 1988;70:33–6.
Schuster DP, Crouch EC, Parks WC, Johnson T, Botney MD. Angiotensin converting enzyme expression in primary pulmonary hypertension. Am J Respir Crit Care Med. 1996;154:1087–91.
Michelakis ED. The role of the NO axis and its therapeutic implications in pulmonary arterial hypertension. Heart Fail Rev. 2003;8:5–21.
Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, et al. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation. 2012;126:963–74.
da Silva GBD, Happé C, Schalij I, Pijacka W, Paton JFR, Guignabert C, et al. Renal denervation reduces pulmonary vascular remodeling and right ventricular diastolic stiffness in experimental pulmonary hypertension. JACC: Bas Trans Sci. 2017;2:22–35.
Shirai M, Tsuchimochi H, Hisashi Nagai H, Gray E, Pearson JT, Sonobe T, et al. Pulmonary vascular tone is dependent on the central modulation of sympathetic nerve activity following chronic intermittent hypoxia. Basic Res Cardiol. 2014;109:432.
Vaillancourt M, Chia P, Sarji S, Nguyen J, Hoftman N, Ruffenach G, et al. Autonomic nervous system involvement in pulmonary arterial hypertension. Respir Res. 2017;18:201.
Velez-Roa S, Ciarka A, Najem B, Vachiery J-L, Naeije R, van de Borne P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation. 2004;110:1308–12.
Kummer W. Pulmonary vascular innervation and its role in responses to hypoxia. Proc Am Thorac Soc. 2011;8:471–6.
Kadowitz PJ, Joiner PD, Hyman AL. Influence of sympathetic stimulation and vasoactive substances on the canine pulmonary veins. J Clin Invest. 1975;56:354–65.
Szidon JP, Flint JF. Significance of sympathetic innervation of pulmonary vessels in response to acute hypoxia. J Appl Physiol. 1977;43:65–71.
• Chen S, Zhang FF, Xu J, Xie DJ, Zhou L, Nguyen T, et al. Pulmonary artery denervation to treat pulmonary arterial hypertension: the single-center, prospective, first-in-man PADN-1 study. J Am Coll Cardiol. 2013;62:1092–100. First-in-man study to test the safety and feasibility pulmonary artery denervation to treat pulmonary hypertension.
Liu Q, Song J, Lu D, Geng J, Jiang Z, Wang K, et al. Effects of renal denervation on monocrotaline induced pulmonary remodeling. Oncotarget. 2017;8:46846–55.
Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–5.
Del Rio R, Moya EA, Iturriaga R. Carotid body and cardiorespiratory alterations in intermittent hypoxia: the oxidative link. Eur Respir J. 2010;36:143–50.
Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992b;20:612–9.
Del Rio R, Moya EA, Iturriaga R. Differential expression of pro-inflammatory cytokines, endothelin-1 and nitric oxide synthases in the rat carotid body exposed to intermittent hypoxia. Brain Res. 2011;1395:74–85.
Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of breathing elicited by chronic intermittent hypoxia. J Appl Physiol. 2003;94:2342–9.
Rey S, Tarvainen MP, Karjalainen PA, Iturriaga R. Dynamic time-varying analysis of heart rate and blood pressure variability in cats exposed to short-term chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2008;295:R28–37.
Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560:577–86.
Lavie L. Obstructive sleep apnoea syndrome: an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51.
Lévy P, Pépin JL, Arnaud C, Tamisier R, Borel JC, Dematteis M, et al. Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J. 2008;32:1082–95.
Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol. 1997;83:95–101.
Del Rio R, Moya EA, Parga MJ, Madrid C, Iturriaga R. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur Respir J. 2012;39:1492–500.
Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, et al. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–10.
Iturriaga R. Translating carotid body function into clinical medicine. J Physiol. 2018;596:3067–77.
Prabhakar NR, Semenza GL. Regulation of carotid body oxygen sensing by hypoxia-inducible factors. Pflugers Arch. 2016;468:71–5.
Moya EA, Arias P, Varela C, Oyarce MP, Del Rio R, Iturriaga R. Intermittent hypoxia-induced carotid body chemosensory potentiation and hypertension are critically dependent on peroxynitrite formation. Oxidative Med Cell Longev. 2016;2016:9802136.
Krause BJ, Casanello P, Dias ACR, Arias P, Velarde V, Arenas GA, et al. Chronic intermittent hypoxia-induced vascular dysfunction in rats is reverted by N-acetylcysteine supplementation and arginase inhibition. Front Physiol. 2018;9:901.
Iturriaga R, Moya EA, Del Rio R. Inflammation and oxidative stress during intermittent hypoxia: the impact on chemoreception. Exp Physiol. 2015;100:149–55.
Del Rio R, Andrade D, Lucero C, Arias P, Iturriaga R. Carotid body ablation abrogates hypertension and autonomic alterations induced by intermittent hypoxia in rats. Hypertension. 2016;68:436–45.
Iturriaga R. Carotid body ablation: a new target to address central autonomic dysfunction. Curr Hyperten Rep. 2018b;20:53.
Iturriaga R, Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Rev. 2004;47:46–53.
Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/ΔFosB in central autonomic regions. Am J Phys. 2011;301:R131–9.
Knight WD, Saxena A, Shell B, Nedungadi P, Mifflin SW, Cunningham JT. Central losartan attenuates increases in arterial pressure and expression of FosB/ΔFosB along the autonomic axis associated with chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1051–8.
Sharpe AL, Calderon AS, Andrade MA, Cunningham JT, Mifflin SW, Toney GM. Chronic intermittent hypoxia increases sympathetic control of blood pressure: role of neuronal activity in the hypothalamic paraventricular nucleus. Am J Phys. 2013;305:H1772–80.
Iturriaga R, Del Rio R, Idiaquez J, Somers VK. Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease. Biol Res Biol Res. 2016;49:13.
Marcus NJ, Philippi NR, Bird CE, Li YL, Schultz HD, Morgan BJ. Effect of AT1 receptor. blockade on intermittent hypoxia -induced endothelial dysfunction. Neurobiol Respir Physiol. 2012;183:67–74.
Kim SJ, Fong AY, Pilowsky PM, Abbott SBG. Sympathoexcitation following intermittent hypoxia in rat is mediated by circulating angiotensin II acting at the carotid body and subfornical organ. J Physiol. 2018;596:3217–32.
Saxena A, Little JT, Nedungadi P, Cunningham JT. Angiotensin II type 1a receptors in subfornical organ contribute towards chronic intermittent hypoxia-associated sustained increase in mean arterial pressure. Am J Phys. 2015;308:H435–46.
• Sugito K, Tatsumi K, Igari H, Kasahara Y, Tani T, Kimura H, et al. Role of carotid body in pressure response of pulmonary circulation in rats. Respir Physiol. 1998;111:283–93. This study shows that carotid body increases arterial blood pressure in the lung.
Shinoda M, Saku K, Abe K, Takehara T, Kuwabara Y, Yoshida K, et al. Carotid body denervation markedly improves the survival of monocrotaline induced pulmonary hypertension rats. The Faseb J. 2015;29:1 suppl.
Funding
This work was supported by grant 1150040 from the National Fund for Scientific and Technological Development of Chile (FONDECYT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pulmonary Hypertension
Rights and permissions
About this article
Cite this article
Iturriaga, R., Castillo-Galán, S. Potential Contribution of Carotid Body-Induced Sympathetic and Renin-Angiotensin System Overflow to Pulmonary Hypertension in Intermittent Hypoxia. Curr Hypertens Rep 21, 89 (2019). https://doi.org/10.1007/s11906-019-0995-y
Published:
DOI: https://doi.org/10.1007/s11906-019-0995-y