Abstract
Purpose of Review
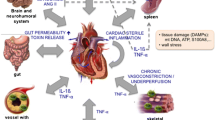
The role of neuroimmune modulation and inflammation in cardiovascular disease has been historically underappreciated. Physiological connections between the heart and brain, termed the heart-brain axis (HBA), are bidirectional, occur through a complex network of autonomic nerves/hormones and cytokines, and play important roles in common disorders.
Recent Findings
At the molecular level, advances in the past two decades reveal complex crosstalk mediated by the sympathetic and parasympathetic nervous systems, the renin-angiotensin aldosterone and hypothalamus-pituitary axes, microRNA, and cytokines. Afferent pathways amplify proinflammatory signals via the hypothalamus and brainstem to the periphery, promoting neurogenic inflammation. At the organ level, while stress-mediated cardiomyopathy is the prototypical disorder of the HBA, cardiac dysfunction can result from a myriad of neurologic insults including stroke and spinal injury. Atrial fibrillation is not necessarily a causative factor for cardioembolic stroke, but a manifestation of an abnormal atrial substrate, which can lead to the development of stroke independent of AF.
Summary
Central and peripheral neurogenic proinflammatory factors have major roles in the HBA, manifesting as complex bi-directional relationships in common conditions such as stroke, arrhythmia, and cardiomyopathy.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annu Rev Immunol. 2018;36:783–812. https://doi.org/10.1146/annurev-immunol-042617-053158.
Pereira VH, Cerqueira JJ, Palha JA, Sousa N. Stressed brain, diseased heart: a review on the pathophysiologic mechanisms of neurocardiology. Int J Cardiol. 2013;166(1):30–7. https://doi.org/10.1016/j.ijcard.2012.03.165.
Rijkers K, Majoie HJ, Hoogland G, Kenis G, De Baets M, Vles JS. The role of interleukin-1 in seizures and epilepsy: a critical review. Exp Neurol. 2009;216(2):258–71. https://doi.org/10.1016/j.expneurol.2008.12.014.
Liu T, Young PR, McDonnell PC, White RF, Barone FC, Feuerstein GZ. Cytokine-induced neutrophil chemoattractant mRNA expressed in cerebral ischemia. Neurosci Lett. 1993;164(1–2):125–8. https://doi.org/10.1016/0304-3940(93)90873-j.
Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, et al. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res. 2007;101(3):304–12. https://doi.org/10.1161/CIRCRESAHA.107.148940.
Scherbakov N, Doehner W. Heart-brain interactions in heart failure. Card Fail Rev. 2018;4(2):87–91. https://doi.org/10.15420/cfr.2018.14.2.
Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, Demkow U. Brain inflammation and hypertension: the chicken or the egg? J Neuroinflammation. 2015;12:85. https://doi.org/10.1186/s12974-015-0306-8.
Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ, et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prev Cardiol. 2020;4:100130. https://doi.org/10.1016/j.ajpc.2020.100130.
Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE, et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401(10384):1293–301. https://doi.org/10.1016/S0140-6736(23)00215-5.
• Badoer E. New insights into the role of inflammation in the brain in heart failure. Front Physiol. 2022;13:837723. https://doi.org/10.3389/fphys.2022.837723. This paper reviews the influence of circulating, proinflammatory cytokines on cardiac function beyond the cardiovascular system, including brain activation of the sympathetic nervous system.
Chan SH, Chan JY. Angiotensin-generated reactive oxygen species in brain and pathogenesis of cardiovascular diseases. Antioxid Redox Signal. 2013;19(10):1074–84. https://doi.org/10.1089/ars.2012.4585.
Paton JF, Waki H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci Biobehav Rev. 2009;33(2):89–94. https://doi.org/10.1016/j.neubiorev.2008.05.020.
Saavedra JM, Angiotensin II. AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin Sci (Lond). 2012;123(10):567–90. https://doi.org/10.1042/CS20120078.
Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation. 2012;9:212. https://doi.org/10.1186/1742-2094-9-212.
de Kloet AD, Krause EG, Shi PD, Zubcevic J, Raizada MK, Sumners C. Neuroimmune communication in hypertension and obesity: a new therapeutic angle? Pharmacol Ther. 2013;138(3):428–40. https://doi.org/10.1016/j.pharmthera.2013.02.005.
Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32(5–6):419–25. https://doi.org/10.1111/j.1440-1681.2005.04205.x.
Esler M. The sympathetic nervous system through the ages: from Thomas Willis to resistant hypertension. Exp Physiol. 2011;96(7):611–22. https://doi.org/10.1113/expphysiol.2010.052332.
Takahashi H. Upregulation of the renin-angiotensin-aldosterone-ouabain system in the brain is the core mechanism in the genesis of all types of hypertension. Int J Hypertens. 2012;2012:242786. https://doi.org/10.1155/2012/242786.
Kasparov S, Teschemacher AG. Altered central catecholaminergic transmission and cardiovascular disease. Exp Physiol. 2008;93(6):725–40. https://doi.org/10.1113/expphysiol.2007.041814.
Szczepanska-Sadowska E, Cudnoch-Jedrzejewska A, Ufnal M, Zera T. Brain and cardiovascular diseases: common neurogenic background of cardiovascular, metabolic and inflammatory diseases. J Physiol Pharmacol. 2010;61(5):509–21.
Muller DN, Mervaala EM, Schmidt F, Park JK, Dechend R, Genersch E, et al. Effect of bosentan on NF-kappaB, inflammation, and tissue factor in angiotensin II-induced end-organ damage. Hypertension. 2000;36(2):282–90. https://doi.org/10.1161/01.hyp.36.2.282.
Paton JF, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med (Berl). 2008;86(6):705–10. https://doi.org/10.1007/s00109-008-0324-4.
Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience. 2010;171(3):852–8. https://doi.org/10.1016/j.neuroscience.2010.09.029.
Guillot FL, Audus KL. Angiotensin peptide regulation of bovine brain microvessel endothelial cell monolayer permeability. J Cardiovasc Pharmacol. 1991;18(2):212–8. https://doi.org/10.1097/00005344-199108000-00006.
Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29(3):640–7. https://doi.org/10.1038/jcbfm.2008.158.
Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63(3):572–9. https://doi.org/10.1161/HYPERTENSIONAHA.113.01743.
Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaB in the paraventricular nucleus. Hypertension. 2012;59(1):113–21. https://doi.org/10.1161/HYPERTENSIONAHA.111.182154.
Felder RB, Yu Y, Zhang ZH, Wei SG. Pharmacological treatment for heart failure: a view from the brain. Clin Pharmacol Ther. 2009;86(2):216–20. https://doi.org/10.1038/clpt.2009.117.
Felder RB. Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp Physiol. 2010;95(1):19–25. https://doi.org/10.1113/expphysiol.2008.045948.
Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289–97. https://doi.org/10.1111/j.1748-1716.2011.02313.x.
Reina-Couto M, Pereira-Terra P, Quelhas-Santos J, Silva-Pereira C, Albino-Teixeira A, Sousa T. Inflammation in human heart failure: major mediators and therapeutic targets. Front Physiol. 2021;12:746494. https://doi.org/10.3389/fphys.2021.746494.
Xu B, Zheng H, Patel KP. Enhanced activation of RVLM-projecting PVN neurons in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2012;302(8):H1700–11. https://doi.org/10.1152/ajpheart.00722.2011.
Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102(25):3060–7. https://doi.org/10.1161/01.cir.102.25.3060.
Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG. Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002;53(4):822–30. https://doi.org/10.1016/s0008-6363(01)00503-x.
Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109(13):1594–602. https://doi.org/10.1161/01.CIR.0000124490.27666.B2.
Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71(21):2392–401. https://doi.org/10.1016/j.jacc.2018.03.002.
Ghaddar B, Diotel N. Zebrafish: a new promise to study the impact of metabolic disorders on the brain. Int J Mol Sci. 2022;23(10). https://doi.org/10.3390/ijms23105372.
Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–42. https://doi.org/10.1001/jama.292.18.2237.
Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab. 2013;2(4):356–63. https://doi.org/10.1016/j.molmet.2013.09.005.
Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, et al. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: data from the Baltimore Longitudinal Study of Aging. PLoS ONE. 2010;5(4):e10099. https://doi.org/10.1371/journal.pone.0010099.
Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96(26):15239–44. https://doi.org/10.1073/pnas.96.26.15239.
• Bhusal A, Rahman MH, Suk K. Hypothalamic inflammation in metabolic disorders and aging. Cell Mol Life Sci. 2021;79(1):32. https://doi.org/10.1007/s00018-021-04019-x. This is a comprehensive review establishing the role of glial cells in the causal relationship between hypothalamic inflammation and the development of metabolic diseases, such as hypertension, obesity, and diabetes.
Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo syndrome. Circulation. 2017;135(24):2426–41. https://doi.org/10.1161/circulationaha.116.027121.
Nef HM, Möllmann H, Akashi YJ, Hamm CW. Mechanisms of stress (Takotsubo) cardiomyopathy. Nat Rev Cardiol. 2010;7(4):187–93. https://doi.org/10.1038/nrcardio.2010.16.
Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain–heart interaction. Circ Res. 2017;121(4):451–68. https://doi.org/10.1161/circresaha.117.311170.
Fure B, Bruun Wyller T, Thommessen B. Electrocardiographic and troponin T changes in acute ischaemic stroke. J Intern Med. 2006;259(6):592–7. https://doi.org/10.1111/j.1365-2796.2006.01639.x.
Khechinashvili G, Asplund K. Electrocardiographic changes in patients with acute stroke: a systematic review. cerebrovascular diseases. 2002;14(2):67–76. https://doi.org/10.1159/000064733.
Yperzeele L, Van Hooff R-J, Nagels G, De Smedt A, De Keyser J, Brouns R. Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic review. Int J Stroke. 2015;10(6):796–800. https://doi.org/10.1111/ijs.12573.
Park H-K, Kim BJ, Yoon C-H, Yang MH, Han M-K, Bae H-J. Left ventricular diastolic dysfunction in ischemic stroke: functional and vascular outcomes. J Stroke. 2016;18(2):195–202. https://doi.org/10.5853/jos.2015.01697.
Prosser J, Macgregor L, Lees KR, Diener H-C, Hacke W, Davis S. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007;38(8):2295–302. https://doi.org/10.1161/strokeaha.106.471813.
Gauberti M, Montagne A, Quenault A, Vivien D. Molecular magnetic resonance imaging of brain-immune interactions. Front Cell Neurosci. 2014;8:389. https://doi.org/10.3389/fncel.2014.00389.
van der Bilt IA, Vendeville JP, van de Hoef TP, Begieneman MP, Lagrand WK, Kros JM, et al. Myocarditis in patients with subarachnoid hemorrhage: a histopathologic study. J Crit Care. 2016;32:196–200. https://doi.org/10.1016/j.jcrc.2015.12.005.
Qiang L, Hong L, Ningfu W, Huaihong C, Jing W. Expression of miR-126 and miR-508-5p in endothelial progenitor cells is associated with the prognosis of chronic heart failure patients. Int J Cardiol. 2013;168(3):2082–8. https://doi.org/10.1016/j.ijcard.2013.01.160.
Wang S, Aurora AB, Johnson BA, Qi X, Mcanally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71. https://doi.org/10.1016/j.devcel.2008.07.002.
Long G, Wang F, Li H, Yin Z, Sandip C, Lou Y, et al. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013;13(1):178. https://doi.org/10.1186/1471-2377-13-178.
Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, et al. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res. 2017;8(4):374–85. https://doi.org/10.1007/s12975-017-0520-z.
Lackner P, Dietmann A, Beer R, Fischer M, Broessner G, Helbok R, et al. Cellular microparticles as a marker for cerebral vasospasm in spontaneous subarachnoid hemorrhage. Stroke. 2010;41(10):2353–7. https://doi.org/10.1161/strokeaha.110.584995.
Schoch B, Regel JP, Wichert M, Gasser T, Volbracht L, Stolke D. Analysis of intrathecal interleukin-6 as a potential predictive factor for vasospasm in subarachnoid hemorrhage. Neurosurgery. 2007;60(5):828–36; discussion -36. https://doi.org/10.1227/01.NEU.0000255440.21495.80.
Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Völker C, et al. Cardiovascular events during World Cup Soccer. N Engl J Med. 2008;358(5):475–83. https://doi.org/10.1056/nejmoa0707427.
Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318(16):1005–12. https://doi.org/10.1056/nejm198804213181601.
Goldberg AD, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation. 1996;94(10):2402–9. https://doi.org/10.1161/01.cir.94.10.2402.
Lazzarino AI, Hamer M, Gaze D, Collinson P, Steptoe A. The association between cortisol response to mental stress and high-sensitivity cardiac troponin T plasma concentration in healthy adults. J Am Coll Cardiol. 2013;62(18):1694–701. https://doi.org/10.1016/j.jacc.2013.05.070.
Wilbert-Lampen U, Nickel T, Leistner D, Guthlin D, Matis T, Volker C, et al. Modified serum profiles of inflammatory and vasoconstrictive factors in patients with emotional stress-induced acute coronary syndrome during World Cup Soccer 2006. J Am Coll Cardiol. 2010;55(7):637–42. https://doi.org/10.1016/j.jacc.2009.07.073.
Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–48. https://doi.org/10.1056/nejmoa043046.
Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14(1):30–8. https://doi.org/10.1038/nrcardio.2016.163.
Freeman R. Cardiovascular manifestations of autonomic epilepsy. Clin Auton Res. 2006;16(1):12–7. https://doi.org/10.1007/s10286-006-0278-y.
Tinuper P, Bisulli F, Cerullo A, Carcangiu R, Marini C, Pierangeli G, et al. Ictal bradycardia in partial epileptic seizures: autonomic investigation in three cases and literature review. Brain. 2001;124(12):2361–71. https://doi.org/10.1093/brain/124.12.2361.
Furlan JC, Fehlings MG. Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurgical Focus FOC. 2008;25(5):E13. https://doi.org/10.3171/FOC.2008.25.11.E13.
Park DY, Hu JR, Alexander KP, Nanna MG. Readmission and adverse outcomes after percutaneous coronary intervention in patients with dementia. J Am Geriatr Soc. 2023;71(4):1034–46. https://doi.org/10.1111/jgs.18120.
Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47(3):895–900. https://doi.org/10.1161/strokeaha.115.012004.
Trayanova NA. Mathematical approaches to understanding and imaging atrial fibrillation: significance for mechanisms and management. Circ Res. 2014;114(9):1516–31. https://doi.org/10.1161/circresaha.114.302240.
Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–47. https://doi.org/10.1161/circulationaha.113.005119.
Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30(10):2019–24. https://doi.org/10.1161/01.str.30.10.2019.
Kamel H, O’Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the atherosclerosis risk in communities study. Ann Neurol. 2015;78(5):670–8. https://doi.org/10.1002/ana.24482.
Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Nazarian S, et al. P-wave morphology and the risk of incident ischemic stroke in the multi-ethnic study of atherosclerosis. Stroke. 2014;45(9):2786–8. https://doi.org/10.1161/strokeaha.114.006364.
Kamel H, Elkind MSV, Bhave PD, Navi BB, Okin PM, Iadecola C, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44(6):1550–4. https://doi.org/10.1161/strokeaha.113.001118.
Larsen BS, Kumarathurai P, Falkenberg J, Nielsen OW, Sajadieh A. Excessive atrial ectopy and short atrial runs increase the risk of stroke beyond incident atrial fibrillation. J Am Coll Cardiol. 2015;66(3):232–41. https://doi.org/10.1016/j.jacc.2015.05.018.
De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89(4):754–65. https://doi.org/10.1093/cvr/cvq357.
Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–9. https://doi.org/10.1056/NEJMoa1105575.
Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, et al. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2-VASc score of 0 or 1? Stroke. 2012;43(10):2551–5. https://doi.org/10.1161/strokeaha.112.667865.
Cai H, Li Z, Goette A, Mera F, Honeycutt C, Feterik K, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106(22):2854–8. https://doi.org/10.1161/01.cir.0000039327.11661.16.
Warraich HJ, Gandhavadi M, Manning WJ. Mechanical discordance of the left atrium and appendage: a novel mechanism of stroke in paroxysmal atrial fibrillation. Stroke. 2014;45(5):1481–4. https://doi.org/10.1161/strokeaha.114.004800.
Sanna T, Diener H-C, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–86. https://doi.org/10.1056/nejmoa1313600.
Budaj A, Flasinska K, Gore JM, Anderson FA Jr, Dabbous OH, Spencer FA, et al. Magnitude of and risk factors for in-hospital and postdischarge stroke in patients with acute coronary syndromes: findings from a Global Registry of Acute Coronary Events. Circulation. 2005;111(24):3242–7. https://doi.org/10.1161/circulationaha.104.512806.
Head SJ, Milojevic M, Daemen J, Ahn JM, Boersma E, Christiansen EH, et al. Stroke rates following surgical versus percutaneous coronary revascularization. J Am Coll Cardiol. 2018;72(4):386–98. https://doi.org/10.1016/j.jacc.2018.04.071.
Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. https://doi.org/10.1161/01.str.24.1.35.
Merkler AE, Diaz I, Wu X, Murthy SB, Gialdini G, Navi BB, et al. Duration of heightened ischemic stroke risk after acute myocardial infarction. J Am Heart Assoc. 2018;7(22). https://doi.org/10.1161/jaha.118.010782.
Kotecha T, Rakhit RD. Acute coronary syndromes. Clin Med (Lond). 2016;16(Suppl 6):s43–8. https://doi.org/10.7861/clinmedicine.16-6-s43.
Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004;286(6):H2264–71. https://doi.org/10.1152/ajpheart.01072.2003.
•• Gelosa P, Castiglioni L, Rzemieniec J, Muluhie M, Camera M, Sironi L. Cerebral derailment after myocardial infarct: mechanisms and effects of the signaling from the ischemic heart to brain. J Mol Med. 2022;100(1):23–41. https://doi.org/10.1007/s00109-021-02154-3. This is a comprehensive review of molecular mechanisms for how cardiac disorders, such as myocardial infarction, can enhance systemic inflammation and alter extracellular vesicles and circulating micro RNAs to cause neurologic dysfunction, including increasing the risk of stroke in MI.
Stellos K, Panagiota V, Kögel A, Leyhe T, Gawaz M, Laske C. Predictive value of platelet activation for the rate of cognitive decline in Alzheimer’s disease patients. J Cereb Blood Flow Metab. 2010;30(11):1817–20. https://doi.org/10.1038/jcbfm.2010.140.
Ozcan Cetin EH, Cetin MS, Aras D, Topaloglu S, Temizhan A, Kisacik HL, et al. Platelet to lymphocyte ratio as a prognostic marker of in-hospital and long-term major adverse cardiovascular events in ST-segment elevation myocardial infarction. Angiology. 2016;67(4):336–45. https://doi.org/10.1177/0003319715591751.
Yang Y, Xie D, Zhang Y. Increased platelet-to-lymphocyte ratio is an independent predictor of hemorrhagic transformation and in-hospital mortality among acute ischemic stroke with large-artery atherosclerosis patients. Int J Gen Med. 2021;14:7545–55. https://doi.org/10.2147/ijgm.S329398.
Deckers K, Schievink SHJ, Rodriquez MMF, Van Oostenbrugge RJ, Van Boxtel MPJ, Verhey FRJ, et al. Coronary heart disease and risk for cognitive impairment or dementia: Systematic review and meta-analysis. PLoS ONE. 2017;12(9):e0184244. https://doi.org/10.1371/journal.pone.0184244.
Kasprzak D, Rzeźniczak J, Ganowicz T, Łuczak T, Słomczyński M, Hiczkiewicz J, et al. A review of acute coronary syndrome and its potential impact on cognitive function. Glob Heart. 2021;16(1):53. https://doi.org/10.5334/gh.934.
Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–713. https://doi.org/10.1161/STR.0b013e3182299496.
Abete P, Della-Morte D, Gargiulo G, Basile C, Langellotto A, Galizia G, et al. Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res Rev. 2014;18:41–52. https://doi.org/10.1016/j.arr.2014.07.003.
Kucheryavykh LY, Davila-Rodriguez J, Rivera-Aponte DE, Zueva LV, Washington AV, Sanabria P, et al. Platelets are responsible for the accumulation of beta-amyloid in blood clots inside and around blood vessels in mouse brain after thrombosis. Brain Res Bull. 2017;128:98–105. https://doi.org/10.1016/j.brainresbull.2016.11.008.
Gagno G, Ferro F, Fluca AL, Janjusevic M, Rossi M, Sinagra G, et al. From brain to heart: possible role of amyloid-β in ischemic heart disease and ischemia-reperfusion injury. Int J Mol Sci. 2020;21(24):9655. https://doi.org/10.3390/ijms21249655.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. https://doi.org/10.1016/s0092-8674(04)00045-5.
Li C, Fang Z, Jiang T, Zhang Q, Liu C, Zhang C, et al. Serum microRNAs profile from genome-wide serves as a fingerprint for diagnosis of acute myocardial infarction and angina pectoris. BMC Med Genomics. 2013;6(1):16. https://doi.org/10.1186/1755-8794-6-16.
Devaux Y, Vausort M, Goretti E, Nazarov PV, Azuaje F, Gilson G, et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem. 2012;58(3):559–67. https://doi.org/10.1373/clinchem.2011.173823.
Boštjančič E, Zidar N, Štajer D, Glavač D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115(3):163–9. https://doi.org/10.1159/000268088.
Ramírez Echeverría MDL, Schoo C, Paul M. Delirium. StatPearls. Treasure Island (FL): StatPearls Copyright © 2022, StatPearls Publishing LLC.; 2022.
Li S, Zhang XH, Zhou GD, Wang JF. Delirium after primary percutaneous coronary intervention in aged individuals with acute ST-segment elevation myocardial infarction: a retrospective study. Exp Ther Med. 2019;17(5):3807–13. https://doi.org/10.3892/etm.2019.7398.
Cortés-Beringola A, Vicent L, Martín-Asenjo R, Puerto E, Domínguez-Pérez L, Maruri R, et al. Diagnosis, prevention, and management of delirium in the intensive cardiac care unit. Am Heart J. 2021;232:164–76. https://doi.org/10.1016/j.ahj.2020.11.011.
Vives-Borrás M, Martínez-Sellés M, Ariza-Solé A, Vidán MT, Formiga F, Bueno H, et al. Clinical and prognostic implications of delirium in elderly patients with non-ST-segment elevation acute coronary syndromes. J Geriatr Cardiol. 2019;16(2):121–8. https://doi.org/10.11909/j.issn.1671-5411.2019.02.008.
Silva M, Pereira E, Rocha A, Sousa D, Peixoto B. Neurocognitive impairment after acute coronary syndrome: prevalence and characterization in a hospital-based cardiac rehabilitation program sample. J Cardiovasc Thorac Res. 2018;10(2):70–5. https://doi.org/10.15171/jcvtr.2018.11.
Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive care unit delirium: a review of diagnosis, prevention, and treatment. Anesthesiology. 2016;125(6):1229–41. https://doi.org/10.1097/aln.0000000000001378.
Sun SH, Yang L, Sun DF, Wu Y, Han J, Liu RC, et al. Effects of vasodilator and esmolol-induced hemodynamic stability on early post-operative cognitive dysfunction in elderly patients: a randomized trial. Afr Health Sci. 2016;16(4):1056–66. https://doi.org/10.4314/ahs.v16i4.23.
• Park DY, Jamil Y, Hu JR, Lowenstern A, Frampton J, Abdullah A, et al. Delirium in older adults after percutaneous coronary intervention: prevalence, risks, and clinical phenotypes. Cardiovasc Revasc Med. 2023. https://doi.org/10.1016/j.carrev.2023.06.010. This paper outlines the risk of delirium and mortality as poor outcomes after percutaneous intervention in older adults with acute coronary syndrome, underscoring the neuroinflammatory response associated with the periprocedural period.
Wang X, Feng K, Liu H, Liu Y, Ye M, Zhao G, et al. Regional cerebral oxygen saturation and postoperative delirium in endovascular surgery: a prospective cohort study. Trials. 2019;20(1):504. https://doi.org/10.1186/s13063-019-3586-y.
Funding
Michael G. Nanna reports research funding from the Patient-Centered Outcomes Research Institute and the American College of Cardiology and grants from Yale Claude D. Pepper Older Americans Independence Center (P30AG021342) and the National Institute on Aging (R03AG074067); none of this funding is related to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michael G. Nanna reports personal fees from Merck and HeartFlow, Inc., outside the submitted work. The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, JR., Abdullah, A., Nanna, M.G. et al. The Brain–Heart Axis: Neuroinflammatory Interactions in Cardiovascular Disease. Curr Cardiol Rep 25, 1745–1758 (2023). https://doi.org/10.1007/s11886-023-01990-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-023-01990-8