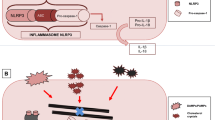
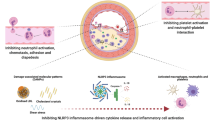
Abstract
Purpose of Review
The pathogenesis and progression of coronary artery disease (CAD) is now known to be largely driven by inflammation on top of the well-accepted role for the disequilibrium between cholesterol deposition and removal from the arterial wall. Recent clinical trials have supported the inflammatory hypothesis of CAD and will be discussed in this review.
Recent Findings
The clinical trial Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) found that treatment with canakinumab, an anti-interleukin-1β agent, resulted in a reduction in non-fatal myocardial infarction, non-fatal stroke, or death. This provided evidence for the inflammatory hypothesis of CAD. However, canakinumab is not cost-effective for widespread therapy and more cost-effective treatments are warranted. The Cardiovascular Inflammation Reduction Trial (CIRT), Colchicine Cardiovascular Outcomes Trial (COLCOT), and Low-Dose Colchicine 2 (LoDoCo2) are recent clinical trials that increased the understanding of the inflammatory hypothesis of CAD.
Summary
Cost-effective therapies targeting inflammation are the future of preventative CAD treatment. Additional clinical trials with anti-inflammatory and anti-cytokine agents would help delineate the most beneficial target for CAD prevention.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Collaborators, G.B.D.C.o.D. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–88.
Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138(5 Pt 2):S419–20.
•• Nissen SE, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352(1):29–38 This study demonstrated that intensive statin therapy results in not only lower atherogenic proteins but also lower CRP. It also showed that the absolute decrease in CRP was independently correlated to progression of CAD.
Yang L, Liu Y, Wang S, Liu T, Cong H. Association between Lp-PLA2 and coronary heart disease in Chinese patients. J Int Med Res. 2017;45(1):159–69.
• Ridker PM, et al. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126(23):2739–48 Findings from this phase IIb study showed that canakinumab reduces inflammation without major effects on cholesterol.
•• Ridker PM, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–8 This study showed that patients with lower CRP levels had better CAD outcomes regardless of level of LDL cholesterol.
Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130.
Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–75.
Xiao L, Liu Y, Wang N. New paradigms in inflammatory signaling in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2014;306(3):H317–25.
Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–4.
Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015;107(3):321–30.
Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114(11):1757–71.
• Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol. 2017;70(18):2278–89 This article outlines the pathophysiology behind interleukins as a target in atherosclerosis.
Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, van Ness K, Greenstreet TA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256(5053):97–100.
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26.
Rajamaki K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5(7):e11765.
Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122(12):1722–40.
Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50.
Dinarello CA, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139(6):1902–10.
Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–147.
Castell JV, Gómez-lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12(5):1179–86.
Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621–36.
Barra NG, Henriksbo BD, Anhê FF, Schertzer JD. The NLRP3 inflammasome regulates adipose tissue metabolism. Biochem J. 2020;477(6):1089–107.
Mallat Z, Corbaz A, Scoazec A, Besnard S, Lesèche G, Chvatchko Y, et al. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104(14):1598–603.
Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162(4):597–605.
•• Ridker PM, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31 This study, CANTOS, demonstrated that anti-interleukin-1beta agent canakinumab resulted in a lower rate of recurrent MACE than placebo, regardless of cholesterol levels.
Rothman AM, MacFadyen J, Thuren T, Webb A, Harrison DG, Guzik TJ, et al. Effects of interleukin-1beta inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension. 2020;75(2):477–82.
Ridker PM, MacFadyen J, Thuren T, Libby P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1beta inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur Heart J. 2019;40.
Sehested TSG, Bjerre J, Ku S, Chang A, Jahansouz A, Owens DK, et al. Cost-effectiveness of Canakinumab for prevention of recurrent cardiovascular events. JAMA Cardiol. 2019;4(2):128–35.
• Ridker PM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial, 10118. Lancet. 2018;391:319–28 This subanalysis of CANTOS showed that participants on canakinumab who achieved an hsCRP concentration of less than 2 mg/L had a 25% reduction in major adverse cardiovascular events and a 31% reduction in cardiovascular mortality and all-cause mortality; however, patients treated with canakinumab who achieved an hsCRP concentration of 2 mg/L or greater did not experience either of these outcomes.
•• Ridker PM, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–62 Findings from this study, CIRT, showed that low-dose methotrexate did not decrease inflammatory markers and did not reduce CAD events compared to placebo.
Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford). 2008;47(3):249–55.
•• Tardif JC, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381(26):2497–505 This study, COLCOT, showed that low-dose colchicine was associated with a statistically significant decrease in the risk of CAD events compared to placebo. However, the absolute decrease in the composite risk was small and was largely driven by a decrease in the risk of stroke.
Shekelle PG, Newberry SJ, FitzGerald JD, Motala A, O'Hanlon CE, Tariq A, et al. Management of gout: a systematic review in support of an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166(1):37–51.
Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation. 2005;112(13):2012–6.
Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther. 2014;36(10):1465–79.
Robertson S, Martínez GJ, Payet CA, Barraclough JY, Celermajer DS, Bursill C, et al. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin Sci (Lond). 2016;130(14):1237–46.
Zhou W, et al. NLRP3: a novel mediator in cardiovascular disease. J Immunol Res. 2018;2018:5702103.
Deftereos S, Giannopoulos G, Angelidis C, Alexopoulos N, Filippatos G, Papoutsidakis N, et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation. 2015;132(15):1395–403.
Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61(4):404–10.
Nidorf SM, Fiolet ATL, Eikelboom JW, Schut A, Opstal TSJ, Bax WA, et al. The effect of low-dose colchicine in patients with stable coronary artery disease: the LoDoCo2 trial rationale, design, and baseline characteristics. Am Heart J. 2019;218:46–56.
•• Nidorf SM, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020; This article describes the results of the study LoDoCo2, a double-blind placebo controlled clinical trial which studied low-dose colchicine for secondary prevention in patients with stable CAD. Results showed a 31% reduction in its primary endpoint in the colchicine group compared to the placebo group; however, baseline and post-treatment inflammatory markers were not measured in this study.
Shah B, Pillinger M, Zhong H, Cronstein B, Xia Y, Lorin JD, et al. Effects of acute colchicine administration prior to percutaneous coronary intervention: COLCHICINE-PCI randomized trial. Circ Cardiovasc Interv. 2020;13(4):e008717.
Ridker PM. From CANTOS to CIRT to COLCOT to clinic: will all atherosclerosis patients soon be treated with combination lipid-lowering and inflammation-inhibiting agents? Circulation. 2020;141(10):787–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest to report.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Boland, J., Long, C. Update on the Inflammatory Hypothesis of Coronary Artery Disease. Curr Cardiol Rep 23, 6 (2021). https://doi.org/10.1007/s11886-020-01439-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-020-01439-2