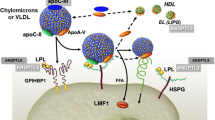
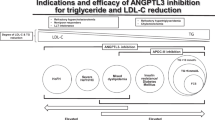
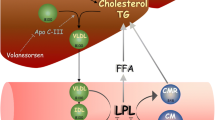
Abstract
Purpose of Review
This review aims to summarize the most recently published literature highlighting the potential of pharmacological inhibition of ANGPTL3 in treating patients suffering from dyslipidemias. The rational for this strategy will be discussed considering evidence describing the role of ANGPTL3 in lipid metabolism and the consequences of its deficiency in humans.
Recent Findings
Recent trials have demonstrated the efficacy and safety of ANGPTL3 inhibition in treating homozygous familial hypercholesterolemia even in those patients carrying biallelic null/null variants, thus supporting the notion that the LDL-lowering effect of ANGPLT3 inhibition is LDLR-independent. The use of ANGPTL3 inhibition strategies has expanded its indications in hypertrygliceridemic patients with functional lipoprotein lipase activity. Contemporarily, the pharmacological research is exploring novel approaches to ANGPTL3 inhibition such as the use of a small interfering RNA targeting the ANGPTL3 transcript in the liver, a protein-based vaccine against ANGPTL3, and a CRISP-Cas-9 method for a liver-selective knock-out of ANGPTL3 gene.
Summary
First, we will describe the molecular function of ANGPTL3 in lipoprotein metabolism. Then, we will revise the clinical characteristics of individuals carrying loss-of-function mutations of ANGPTL3, a rare condition known as familial hypobetalipoproteinemia type 2 (FHBL2) that represents a unique human model of ANGPTL3 deficiency. Finally, we will examine the lipid-lowering potential of pharmacological inhibition of ANGPTL3 based on the results of clinical trials employing Evinacumab, the first approved fully humanized monoclonal antibody against ANGPTL3. The future perspectives for ANGPTL3 inhibition will also be revised.
Graphical Abstract


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289–97. https://doi.org/10.1001/JAMA.2016.13985.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290(1):140–205. https://doi.org/10.1016/J.ATHEROSCLEROSIS.2019.08.014.
Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet (London, England). 2017;390(10106):1962–71. https://doi.org/10.1016/S0140-6736(17)32290-0.
Thedrez A, Blom DJ, Ramin-Mangata S, et al. Homozygous familial hypercholesterolemia patients with identical mutations variably express the LDLR (low-density lipoprotein receptor): implications for the efficacy of Evolocumab. Arterioscler Thromb Vasc Biol. 2018;38(3):592–8. https://doi.org/10.1161/ATVBAHA.117.310217.
Bansal S, Ruzza A, Sawhney JPS, et al. Evolocumab in patients with homozygous familial hypercholesterolemia in India. J Clin Lipidol. 2021;15(6):814–21. https://doi.org/10.1016/J.JACL.2021.10.003.
Sniderman AD, Thanassoulis G, Glavinovic T, et al. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. 2019;4(12):1287–95. https://doi.org/10.1001/JAMACARDIO.2019.3780.
Pinzon Grimaldos A, Bini S, Pacella I, et al. The role of lipid metabolism in shaping the expansion and the function of regulatory T cells. Clin Exp Immunol. 2022;208(2):181–92. https://doi.org/10.1093/cei/uxab033.
Feingold KR. Introduction to Lipids and Lipoproteins. MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK305896/. Accessed August 29, 2021.
Björnson E, Adiels M, Taskinen MR, Borén J. Kinetics of plasma triglycerides in abdominal obesity. Curr Opin Lipidol. 2017;28(1):11–8. https://doi.org/10.1097/MOL.0000000000000375.
Borén J, Taskinen MR, Björnson E, Packard CJ. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat Rev Cardiol. 2022. https://doi.org/10.1038/S41569-022-00676-Y.
Klop B, Proctor SD, Mamo JC, Botham KM, Castro Cabezas M. Understanding postprandial inflammation and its relationship to lifestyle behaviour and metabolic diseases. Int J Vasc Med. 2012;2012. https://doi.org/10.1155/2012/947417.
Lopez-Miranda J, Williams C, Larion D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr. 2007;98(3):458–73. https://doi.org/10.1017/S000711450774268X.
Berry SE, Valdes AM, Drew DA, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26(6):964–73. https://doi.org/10.1038/S41591-020-0934-0.
Zhang R, Zhang K. An updated ANGPTL3–4–8 model as a mechanism of triglyceride partitioning between fat and oxidative tissues. Prog Lipid Res. 2021;85. https://doi.org/10.1016/J.PLIPRES.2021.101140.
Bini S, D’Erasmo L, Di Costanzo A, Minicocci I, Pecce V, Arca M. The interplay between angiopoietin-like proteins and adipose tissue: another piece of the relationship between adiposopathy and cardiometabolic diseases? Int J Mol Sci. 2021;22(2):1–16. https://doi.org/10.3390/ijms22020742.
Tikkanen E, Minicocci I, Hällfors J, et al. Metabolomic signature of angiopoietin-like protein 3 deficiency in fasting and postprandial state. Arterioscler Thromb Vasc Biol. 2019;39(4):665–74. https://doi.org/10.1161/ATVBAHA.118.312021.
Banfi S, Gusarova V, Gromada J, et al. Increased thermogenesis by a noncanonical pathway in ANGPTL3/8-deficient mice. https://doi.org/10.1073/pnas.1717420115.
Minicocci I, Montali A, Robciuc MR, et al. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J Clin Endocrinol Metab. 2012;97(7):E1266–75. https://doi.org/10.1210/jc.2012-1298.
Conklin D, Gilbertson D, Taft DW, et al. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics. 1999;62(3):477–82. https://doi.org/10.1006/GENO.1999.6041.
Quagliarini F, Wang Y, Kozlitina J, et al. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci USA. 2012;109(48):19751–6. https://doi.org/10.1073/PNAS.1217552109.
Inaba T, Matsuda M, Shimamura M, et al. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. J Biol Chem. 2003;278(24):21344–51. https://doi.org/10.1074/jbc.M213202200.
Li H, Liu J. The novel function of HINFP as a co-activator in sterol-regulated transcription of PCSK9 in HepG2 cells. Biochem J. 2012;443(3):757–68. https://doi.org/10.1042/BJ20111645.
Minicocci I, Tikka A, Poggiogalle E, et al. Effects of angiopoietin-like protein 3 deficiency on postprandial lipid and lipoprotein metabolism. J Lipid Res. 2016;57(6):1097–107. https://doi.org/10.1194/jlr.P066183.
Li Y, Sun L, Xu H, et al. Angiopoietin-like protein 3 modulates barrier properties of human glomerular endothelial cells through a possible signaling pathway involving phosphatidylinositol-3 kinase/protein kinase B and integrin alphaVbeta3. Acta Biochim Biophys Sin (Shanghai). 2008;40(6):459–65. https://doi.org/10.1111/J.1745-7270.2008.00421.X.
Shimamura M, Matsuda M, Kobayashi S, et al. Angiopoietin-like protein 3, a hepatic secretory factor, activates lipolysis in adipocytes. Biochem Biophys Res Commun. 2003;301(2):604–9. https://doi.org/10.1016/S0006-291X(02)03058-9.
Ono M, Shimizugawa T, Shimamura M, et al. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J Biol Chem. 2003;278(43):41804–9. https://doi.org/10.1074/jbc.M302861200.
Chen YQ, Pottanat TG, Siegel RW, et al. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J Lipid Res. 2020;61(317):jlr.RA120000781. https://doi.org/10.1194/jlr.ra120000781.
Camenisch G, Pisabarro MT, Sherman D, et al. ANGPTL3 Stimulates endothelial cell adhesion and migration via integrin αvβ3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277(19):17281–90. https://doi.org/10.1074/JBC.M109768200.
Bini S, Pecce V, Di Costanzo A, et al. The fibrinogen-like domain of ANGPTL3 facilitates lipolysis in 3T3-L1 cells by activating the intracellular erk pathway. Biomolecules. 2022;12(4):585. https://doi.org/10.3390/biom12040585.
Fujimoto K, Koishi R, Shimizugawa T, Ando Y. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp Anim. 2006;55(1):27–34. https://doi.org/10.1538/EXPANIM.55.27.
Wang Y, Gusarova V, Banfi S, Gromada J, Cohen JC, Hobbs HH. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. J Lipid Res. 2015;56(7):1296–307. https://doi.org/10.1194/JLR.M054882.
Wang Y, McNutt MC, Banfi S, et al. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc Natl Acad Sci U S A. 2015;112(37):11630–5. https://doi.org/10.1073/pnas.1515374112.
Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40(2):189–97. https://doi.org/10.1038/NG.75.
Musunuru K, Pirruccello JP, Do R, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363:2220–7. https://doi.org/10.1056/NEJMoa1002926.
Minicocci I, Santini S, Cantisani V, et al. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: a pooled analysis. J Lipid Res. 2013;54(12):3481–90. https://doi.org/10.1194/jlr.P039875.
Fazio S, Sidoli A, Vivenzio A, et al. A form of familial hypobetalipoproteinaemia not due to a mutation in the apolipoprotein B gene. J Intern Med. 1991;229(1):41–7. https://doi.org/10.1111/J.1365-2796.1991.TB00304.X.
Arca M, D’Erasmo L, Minicocci I. Familial combined hypolipidemia : ANGPTL3 deficiency. Curr Opin Lipidol. 2020;31(2):41–8. https://doi.org/10.1097/MOL.0000000000000668.
Di Costanzo A, Di Leo E, Noto D, et al. Clinical and biochemical characteristics of individuals with low cholesterol syndromes: a comparison between familial hypobetalipoproteinemia and familial combined hypolipidemia. J Clin Lipidol. 2017;11(5):1234–42. https://doi.org/10.1016/j.jacl.2017.06.013.
D’Erasmo L, Neufeld T, Di Martino M, et al. The impact of ANGPTL3 deficiency on hepatic steatosis: Observations from carriers of loss-of-function mutations. Atherosclerosis. 2020;315: e17. https://doi.org/10.1016/J.ATHEROSCLEROSIS.2020.10.064.
Stitziel NO, Khera AV, Wang X, et al. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol. 2017;69(16):2054–63. https://doi.org/10.1016/j.jacc.2017.02.030.
Dewey FE, Gusarova V, Dunbar RL, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377(3):211–21. https://doi.org/10.1056/nejmoa1612790.
Gusarova V, Alexa CA, Wang Y, et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J Lipid Res. 2015;56(7):1308–17. https://doi.org/10.1194/JLR.M054890.
•• Ahmad Z, Banerjee P, Hamon S, et al. Inhibition of angiopoietin-like protein 3 with a monoclonal antibody reduces triglycerides in hypertriglyceridemia. Circulation. 2019;140(6):470–86. https://doi.org/10.1161/CIRCULATIONAHA.118.039107. This paper shows the results of 2 Phase 1 studies proving the efficacy and safety of in hypertriglyceridemic subjects showing comparable results that that observed in carrying loss of function mutations in ANGPTL3.
•• Raal FJ, Rosenson RS, Reeskamp LF, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383(8):711–20. https://doi.org/10.1056/nejmoa2004215. This Phase III trials showed that homozygous familial hypercholesterolemia receiving maximum doses of lipid-lowering therapy plus Evinacumab had a 49% reduction from baseline in LDL-C at 24 weeks as compared the small increase in the placebo group.
• Reeskamp LF, Millar JS, Wu L, et al. ANGPTL3 inhibition with evinacumab results in faster clearance of IDL and LDL apoB in patients with homozygous familial hypercholesterolemia-brief report. Arterioscler Thromb Vasc Biol. 2021;41(5):1753–9. https://doi.org/10.1161/ATVBAHA.120.315204. In this small kinetic study, ANGPTL3 inhibition with evinacumab associates with an increase in the fractional catabolic rate of IDL apoB and LDL apoB.
Evkeeza: Pending EC decision | European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/evkeeza. Accessed June 7, 2021.
Rosenson RS, Burgess LJ, Ebenbichler CF, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383(24):2307–19. https://doi.org/10.1056/nejmoa2031049.
Prakash TP, Graham MJ, Yu J, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42(13):8796–807. https://doi.org/10.1093/NAR/GKU531.
Wang Y, Yu RZ, Henry S, Geary RS. Pharmacokinetics and clinical pharmacology considerations of GalNAc 3-conjugated antisense oligonucleotides. Expert Opin Drug Metab Toxicol. 2019;15(6):475–85. https://doi.org/10.1080/17425255.2019.1621838.
Foss-Freitas MC, Akinci B, Neidert A, et al. Selective targeting of angiopoietin-like 3 (ANGPTL3) with vupanorsen for the treatment of patients with familial partial lipodystrophy (FPLD): results of a proof-of-concept study. Lipids Health Dis. 2021;20(1). https://doi.org/10.1186/S12944-021-01589-4.
Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, Hurh E, Kingsbury J, Bartlett VJ, Figueroa AL, Piscitelli P, Singleton W, Witztum JL, Geary RS, Tsimikas S. Louis St. L O'Dea, the Vupanorsen Study Investigators, Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41(40):3936–3945. https://doi.org/10.1093/eurheartj/ehaa689.
Bergmark BA, Marston NA, Bramson CR, et al. Effect of vupanorsen on non-high-density lipoprotein cholesterol levels in statin-treated patients with elevated cholesterol: TRANSLATE-TIMI 70. Circulation. 2022;145(18):1377–86. https://doi.org/10.1161/CIRCULATIONAHA.122.059266.
Xu YX, Redon V, Yu H, et al. Role of angiopoietin-like 3 (ANGPTL3) in regulating plasma level of low-density lipoprotein cholesterol. Atherosclerosis. 2018;268:196–206. https://doi.org/10.1016/j.atherosclerosis.2017.08.031.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30. https://doi.org/10.1056/nejmoa1913805.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa1912387.
Fukami H, Morinaga J, Nakagami H, et al. Vaccine targeting ANGPTL3 ameliorates dyslipidemia and associated diseases in mouse models of obese dyslipidemia and familial hypercholesterolemia. Cell Rep Med. 2021;2(11). https://doi.org/10.1016/J.XCRM.2021.100446.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. https://doi.org/10.1126/SCIENCE.1225829.
Zalatan JG, Lee ME, Almeida R, et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell. 2015;160(1–2):339–50. https://doi.org/10.1016/J.CELL.2014.11.052.
Chadwick AC, Evitt NH, Lv W, Musunuru K. Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation. 2018;137(9):975–7. https://doi.org/10.1161/CIRCULATIONAHA.117.031335.
D’Erasmo L, Bini S, Arca M. Rare treatments for rare dyslipidemias: new perspectives in the treatment of homozygous familial hypercholesterolemia (HoFH) and familial chylomicronemia syndrome (FCS). Curr Atheroscler Rep. 2021;23(11):65. https://doi.org/10.1007/s11883-021-00967-8.
D’Erasmo L, Minicocci I, Nicolucci A, et al. Autosomal recessive hypercholesterolemia: long-term cardiovascular outcomes. J Am Coll Cardiol. 2018;71(3):279–88. https://doi.org/10.1016/j.jacc.2017.11.028.
Moulin P, Dufour R, Averna M, et al. Identification and diagnosis of patients with familial chylomicronaemia syndrome (FCS): Expert panel recommendations and proposal of an “FCS score.” Atherosclerosis. 2018;275:265–72. https://doi.org/10.1016/J.ATHEROSCLEROSIS.2018.06.814.
D’Erasmo L, Di Costanzo A, Cassandra F, et al. Spectrum of mutations and long-term clinical outcomes in genetic chylomicronemia syndromes. Arterioscler Thromb Vasc Biol. 2019;39(12):2531–41. https://doi.org/10.1161/ATVBAHA.119.313401.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Laura D’Erasmo has received personal fees for public speaking, consultancy or grant support from Amryt Pharmaceuticals, Akcea Therapeutics, Pfizer, Amgen, SOBI and Sanofi.
Marcello Arca has received research grant support from Amryt Pharmaceutical, Amgen, IONIS, Akcea Therapeutics, Pfizer and Sanofi; has served as a consultant for Amgen, Aegerion, Akcea Therapeutics, Regeneron, Sanofi and Alfasigma and received lecturing fees from Amgen, Amryt Pharmaceutical, Pfizer, Sanofi and AlfaSigma.
Simone Bini has received personal fees for public speaking from Akcea Therapeutics and SOBI.
The other authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bini, S., Tramontano, D., Minicocci, I. et al. How ANGPTL3 Inhibition Will Help Our Clinical Practice?. Curr Atheroscler Rep 25, 19–29 (2023). https://doi.org/10.1007/s11883-022-01076-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-022-01076-w