Abstract
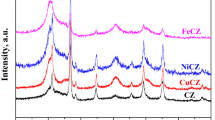
This study examined the catalytic activity and stability of transition metal@C (carbon) catalysts in methane pyrolysis for hydrogen and solid carbon production. The carbon support for the catalysts was sustainably synthesized using CO2 as the carbon source. X-ray diffraction analysis was used to confirm the presence of metallic phases in the as-calcined catalysts without requiring an additional H2 reduction step. The apparent activation energies of the catalysts were determined using Arrhenius plots, with Ni@C having the lowest value (71 kJ∙mol−1), followed by Co@C (89 kJ∙mol−1), Fe@C (100 kJ∙mol−1), and Cu@C (122 kJ∙mol−1). The carbon support exhibited an apparent activation energy of 150 kJ∙mol−1, indicating its superior catalytic performance compared with traditional carbon-based catalysts. The reaction order demonstrated first-order reactions, indicating that the rate-determining step is associated with the first C–H bond cleavage in methane. The Ni@C and Co@C catalysts demonstrated promising catalytic activity and stability for methane pyrolysis, with the formation of crystalline carbon and metal particle fragmentation playing crucial roles in enhancing their performance. However, the formation of carbide species contributed to the deactivation of Fe@C.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
M. Crippa, D. Guizzardi, M. Banja, E. Solazzo, M. Muntean, E. Schaaf, F. Pagani, F. Monforti-Ferrario, J.G.J. Olivier, R. Quadrelli, CO2 emissions of all world countries. JRC Sci. for Policy Report, European Commission, EUR. (2022). https://doi.org/10.2760/07904
C. Le Quéré, G.P. Peters, P. Friedlingstein, R.M. Andrew, J.G. Canadell, S.J. Davis, R.B. Jackson, M.W. Jones, Fossil CO2 emissions in the post-COVID-19 era. Nat. Clim. Chang. 11, 197–199 (2021). https://doi.org/10.1038/s41558-021-01001-0
A. Pareek, R. Dom, J. Gupta, J. Chandran, V. Adepu, P.H. Borse, Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 3, 319–327 (2020). https://doi.org/10.1016/j.mset.2019.12.002
M.J. Chae, J.H. Kim, B. Moon, S. Park, Y.S. Lee, The present condition and outlook for hydrogen-natural gas blending technology. Korean J. Chem. Eng. 39, 251–262 (2022). https://doi.org/10.1007/s11814-021-0960-8
W.S. Chai, Y. Bao, P. Jin, G. Tang, L. Zhou, A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. and Sustainable Energy Rev. 147, 111254 (2021). https://doi.org/10.1016/j.rser.2021.111254
S. Park, Y. Shin, E. Jeong, M. Han, Techno-economic analysis of green and blue hybrid processes for ammonia production. Korean J. Chem. Eng. (2023). https://doi.org/10.1007/s11814-023-1520-1
T.A. Le, Q.C. Do, Y. Kim, T.-W. Kim, H.-J. Chae, A review on the recent developments of ruthenium and nickel catalysts for COx-free H2 generation by ammonia decomposition. Korean J. Chem. Eng. 38, 1087–1103 (2021). https://doi.org/10.1007/s11814-021-0767-7
M. Amin, H.H. Shah, A.G. Fareed, W.U. Khan, E. Chung, A. Zia, Z.U. Rahman Farooqi, C. Lee, Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 47, 33112–33134 (2022). https://doi.org/10.1016/j.ijhydene.2022.07.172
R.S. El-Emam, H. Özcan, Comprehensive review on the techno-economics of sustainable large-scale clean hydrogen production. J. Clean. Prod. 220, 593–609 (2019). https://doi.org/10.1016/j.jclepro.2019.01.309
M. Hermesmann, T.E. Müller, Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 90, 100996 (2022). https://doi.org/10.1016/j.pecs.2022.100996
R.W. Howarth, M.Z. Jacobson, How green is blue hydrogen? Energy Sci. Eng. 9, 1676–1687 (2021). https://doi.org/10.1002/ese3.956
M. Yu, K. Wang, H. Vredenburg, Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrogen Energy 46, 21261–21273 (2021). https://doi.org/10.1016/j.ijhydene.2021.04.016
B. Parkinson, P. Balcombe, J.F. Speirs, A.D. Hawkes, K. Hellgardt, Levelized cost of CO 2 mitigation from hydrogen production routes. Energy Environ. Sci. (2019). https://doi.org/10.1039/c8ee02079e
L. Alves, V. Pereira, T. Lagarteira, A. Mendes, Catalytic methane decomposition to boost the energy transition: Scientific and technological advancements. Renew. and Sustainable Energy Rev. 137, 110465 (2021). https://doi.org/10.1016/j.rser.2020.110465
B. Parkinson, M. Tabatabaei, D.C. Upham, B. Ballinger, C. Greig, S. Smart, E. McFarland, Hydrogen production using methane: Techno-economics of decarbonizing fuels and chemicals. Int. J. Hydrogen Energy 43, 2540–2555 (2018). https://doi.org/10.1016/j.ijhydene.2017.12.081
J. Boo, E.H. Ko, N.K. Park, C. Ryu, Y.H. Kim, J. Park, D. Kang, Methane pyrolysis in molten potassium chloride: An experimental and economic analysis. Energies (Basel) (2021). https://doi.org/10.3390/en14238182
D. Kang, N. Rahimi, M.J. Gordon, H. Metiu, E.W. McFarland, Catalytic methane pyrolysis in molten MnCl2-KCl. Appl. Catal. B 254, 659–666 (2019). https://doi.org/10.1016/j.apcatb.2019.05.026
H.F. Abbas, W.M.A. Wan Daud, Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 35, 1160–1190 (2010). https://doi.org/10.1016/j.ijhydene.2009.11.036
Y. Li, D. Li, G. Wang, Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 162, 1–48 (2011). https://doi.org/10.1016/j.cattod.2010.12.042
Z. Fan, W. Weng, J. Zhou, D. Gu, W. Xiao, Catalytic decomposition of methane to produce hydrogen: A review. J. Energy Chem. 58, 415–430 (2021)
D. Kang, C. Palmer, D. Mannini, N. Rahimi, M.J. Gordon, H. Metiu, E.W. McFarland, Catalytic methane pyrolysis in molten alkali chloride salts containing iron. ACS Catal. 10, 7032–7042 (2020). https://doi.org/10.1021/acscatal.0c01262
C. Su, K.P. Loh, Carbocatalysts: Graphene oxide and its derivatives. Acc. Chem. Res. 46, 2275–2285 (2013). https://doi.org/10.1021/ar300118v
N. Muradov, F. SmithT-Raissi, A, Catalytic activity of carbons for methane decomposition reaction. Catal. Today (2005). https://doi.org/10.1016/j.cattod.2005.02.018
K. Otsuka, H. Ogihara, S. Takenaka, Decomposition of methane over Ni catalysts supported on carbon fibers formed from different hydrocarbons. Carbon N Y. 41, 223–233 (2003). https://doi.org/10.1016/S0008-6223(02)00308-1
Z. Bai, H. Chen, B. Li, W. Li, Methane decomposition over Ni loaded activated carbon for hydrogen production and the formation of filamentous carbon. Int. J. Hydrogen Energy 32, 32–37 (2007). https://doi.org/10.1016/j.ijhydene.2006.06.030
M. Szymańska, A. Malaika, P. Rechnia, A. Miklaszewska, M. Kozłowski, Metal/activated carbon systems as catalysts of methane decomposition reaction. Catal. Today 249, 94–102 (2015). https://doi.org/10.1016/j.cattod.2014.11.025
J. Zhang, W. Xie, X. Li, Q. Hao, H. Chen, X. Ma, Methane decomposition over Ni/carbon catalysts prepared by selective gasification of coal char. Energy Convers. Manag. 177, 330–338 (2018). https://doi.org/10.1016/j.enconman.2018.09.075
Y. Wang, Y. Zhang, S. Zhao, J. Zhu, L. Jin, H. Hu, Preparation of bimetallic catalysts Ni-Co and Ni-Fe supported on activated carbon for methane decomposition. Carbon Resources Conversion 3, 190–197 (2020). https://doi.org/10.1016/j.crcon.2020.12.002
X. Yang, E. Yang, B. Hu, J. Yan, F. Shangguan, Q. Hao, H. Chen, J. Zhang, X. Ma, Nanofabrication of Ni-incorporated three-dimensional ordered mesoporous carbon for catalytic methane decomposition. J. Environ. Chem. Eng. 10, 107451 (2022). https://doi.org/10.1016/j.jece.2022.107451
D. Kang, J.W. Lee, Enhanced methane decomposition over nickel–carbon–B2O3 core–shell catalysts derived from carbon dioxide. Appl. Catal. B 186, 41–55 (2016). https://doi.org/10.1016/j.apcatb.2015.12.045
J. Zhang, J.W. Lee, Production of boron-doped porous carbon by the reaction of carbon dioxide with sodium borohydride at atmospheric pressure. Carbon N Y 53, 216–221 (2013). https://doi.org/10.1016/j.carbon.2012.10.051
B. Fubini, M. Ghiazza, I. Fenoglio, Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology 4, 347–363 (2010). https://doi.org/10.3109/17435390.2010.509519
M. Pumera, A. Ambrosi, E.L.K. Chng, Impurities in graphenes and carbon nanotubes and their influence on the redox properties. Chem. Sci. 3, 3347–3355 (2012). https://doi.org/10.1039/C2SC21374E
W. Kiciński, S. Dyjak, Transition metal impurities in carbon-based materials: Pitfalls, artifacts and deleterious effects. Carbon N Y 168, 748–845 (2020). https://doi.org/10.1016/j.carbon.2020.06.004
J. Zhang, A. Byeon, J.W. Lee, Boron-doped carbon–iron nanocomposites as efficient oxygen reduction electrocatalysts derived from carbon dioxide. Chem. Commun. 50, 6349–6352 (2014). https://doi.org/10.1039/C4CC01903B
J.C. Goak, C.J. Lim, Y. Hyun, E. Cho, Y. Seo, N. Lee, Efficient gas-phase purification using chloroform for metal-free multi-walled carbon nanotubes. Carbon N Y 148, 258–266 (2019). https://doi.org/10.1016/j.carbon.2019.03.077
J.S. Stefano, D.P. Rocha, R.M. Dornellas, L.C.D. Narciso, S.R. Krzyzaniak, P.A. Mello, E. Nossol, E.M. Richter, R.A.A. Munoz, Highly sensitive amperometric detection of drugs and antioxidants on non-functionalized multi-walled carbon nanotubes: Effect of metallic impurities? Electrochim. Acta 240, 80–89 (2017). https://doi.org/10.1016/j.electacta.2017.04.050
G.M. Kim, S. Baik, J.W. Lee, Enhanced oxygen reduction from the insertion of cobalt into nitrogen-doped porous carbons. RSC Adv. 5, 87971–87980 (2015). https://doi.org/10.1039/C5RA15635A
M.H. Kim, E.K. Lee, J.H. Jun, S.J. Kong, G.Y. Han, B.K. Lee, T.-J. Lee, K.J. Yoon, Hydrogen production by catalytic decomposition of methane over activated carbons: kinetic study. Int. J. Hydrogen Energy 29, 187–193 (2004). https://doi.org/10.1016/S0360-3199(03)00111-3
H. Nishii, D. Miyamoto, Y. Umeda, H. Hamaguchi, M. Suzuki, T. Tanimoto, T. Harigai, H. Takikawa, Y. Suda, Catalytic activity of several carbons with different structures for methane decomposition and by-produced carbons. Appl. Surf. Sci. 473, 291–297 (2019). https://doi.org/10.1016/j.apsusc.2018.12.073
S.H. Sharif Zein, A.R. Mohamed, P.S. Talpa Sai, Kinetic studies on catalytic decomposition of methane to hydrogen and carbon over Ni/TiO2 catalyst. Ind. Eng. Chem. Res. 43, 4864–4870 (2004). https://doi.org/10.1021/ie034208f
D. Bae, Y. Kim, E.H. Ko, S. Ju Han, J.W. Lee, M. Kim, D. Kang, Methane pyrolysis and carbon formation mechanisms in molten manganese chloride mixtures. Appl. Energy 336, 120810 (2023). https://doi.org/10.1016/j.apenergy.2023.120810
Z. Bai, H. Chen, B. Li, W. Li, Catalytic decomposition of methane over activated carbon. J. Anal. Appl. Pyrolysis 73, 335–341 (2005). https://doi.org/10.1016/j.jaap.2005.03.004
Z. Bai, H. Chen, W. Li, B. Li, Hydrogen production by methane decomposition over coal char. Int. J. Hydrogen Energy 31, 899–905 (2006). https://doi.org/10.1016/j.ijhydene.2005.08.001
C.H. Bartholomew, Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 212, 17–60 (2001). https://doi.org/10.1016/S0926-860X(00)00843-7
U.P.M. Ashik, W.M.A.W. Daud, Probing the differential methane decomposition behaviors of n-Ni/SiO2, n-Fe/SiO2 and n-Co/SiO2 catalysts prepared by co-precipitation cum modified Stöber method. RSC Adv. 5, 67227–67241 (2015). https://doi.org/10.1039/C5RA10997C
J.L. Pinilla, R. Utrilla, M.J. Lázaro, R. Moliner, I. Suelves, A.B. García, Ni- and Fe-based catalysts for hydrogen and carbon nanofilament production by catalytic decomposition of methane in a rotary bed reactor. Fuel Process. Technol. 92, 1480–1488 (2011). https://doi.org/10.1016/j.fuproc.2011.03.009
L. Zhou, Y. Guo, K. Hideo, Unsupported nickel catalysts for methane catalytic decomposition into pure hydrogen. AIChE J. 60, 2907–2917 (2014). https://doi.org/10.1002/aic.14487
Acknowledgements
This study was financially supported by Seoul National University of Science and Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, M., Lyu, J., Lee, J.W. et al. Catalytic Activity of CO2-Derived Transition Metal–Carbon Catalysts in Methane Pyrolysis. Korean J. Chem. Eng. 41, 1479–1490 (2024). https://doi.org/10.1007/s11814-024-00097-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00097-2