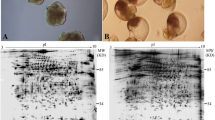
Abstract
Amphibian metamorphosis is a tightly regulated transformation involving the participation of hormones and other biomolecules in cell death and tail absorption. Among the regulators, phosphate is essential for various processes, such as cell death and survival and enzyme activity regulation. Therefore, identification and characterization of phosphatases in L. catesbeianus tail may contribute to understand the events involved in cell death and nutrient release during metamorphosis. Differential centrifugation was used to separate soluble proteins from membrane proteins and analyzed by phosphomonohydrolases, serine/threonine protein phosphatase 2A (PP2A), and protein tyrosine phosphatase (PTP) assays. Mitochondrial fractioning was used to evaluate PP2A and alkaline phosphatase activities. Tandem mass spectrometry (MS/MS) analysis was performed using the crude extract. Phosphomonohydrolase activity was assayed by p-nitrophenylphosphate (pNPP) hydrolysis, whereas PP2A and PTP were assayed by peptides phosphorylated in threonine and tyrosine, respectively; inhibitor-2 was used to identify the serine/threonine protein phosphatase type 1 (PP1). The enzymatic activities and kinetic parameters of pNPP hydrolysis revealed three distinct phosphomonohydrolases. MS/MS analysis of the crude extract revealed three protein phosphatases, viz., PP2A, PP1, and a PTP, which was confirmed by in vitro assays. The results may relate PP2A activity to membrane-bound alkaline phosphatase, PTP activity to soluble acid phosphatase, and PP1 activity to membrane acid phosphatase, although more detailed studies are needed to confirm this hypothesis. We propose a model providing information on the role of PP1 and PP2A in the signaling events leading to cell death and the role of these enzymes in anuran tail absorption during metamorphosis.





Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- BAD:
-
BCL-2 antagonist of cell death
- BAX:
-
BCL-2 Associated X
- BCL-2:
-
B cell lymphoma 2 – family of regulator proteins
- BCL-XL:
-
B cell lymphoma-extra large
- BSA:
-
Bovine serum albumin
- ESI:
-
Electrospray ionization
- FWHM:
-
Full Width at Half Maximum
- GPI:
-
Glycosylphosphatidylinositol
- HCD:
-
Higher-energy C-trap dissociation
- IL-2:
-
Interleukin-2
- LMW-PTP:
-
Low molecular weight phosphotyrosine protein phosphatase
- MIT:
-
Mitochondrial fraction
- MOMP:
-
Mitochondrial outer membrane permeabilization
- PDGF:
-
Platelet derived growth factor
- PIPLC:
-
Phospholipase C
- pNPP:
-
p-nitrophenylphosphate
- PPP:
-
Phosphoprotein phosphatases
- PP1:
-
Protein phosphatase 1
- PP2A:
-
Protein phosphatase 2A
- PP2B:
-
Protein phosphatase 2B
- PP4:
-
Protein phosphatase 4
- PP5:
-
Protein phosphatase 5
- PP6:
-
Protein phosphatase 6
- PP7:
-
Protein phosphatase 7
- PPM:
-
Magnesium dependent protein phosphatases
- PSMs:
-
Peptide-spectrum matches
- PTP:
-
Protein tyrosine phosphatase
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SMP:
-
Fraction containing soluble and cytoplasmic membrane proteins
- TH:
-
Thyroid hormone
- TNF:
-
Tumor necrosis factor
References
Altig R (2007) A primer for the morphology of anuran tadpoles. Herpetol Conserv Biol 2(1):71–74 Retrieved from http://www.herpconbio.org/Volume_2/Issue_1/Altig_2007b.pdf
Bredesen DE, Rao RV, Mehlen P (2006) Cell death in the nervous system. Nature 443:796–802. https://doi.org/10.1038/nature05293
Brown DD, Cai L (2007) Amphibian metamorphosis. Dev Biol 306:22–33. https://doi.org/10.1016/j.ydbio.2007.03.021
Candiano G, Bruschi M, Musante L et al (2004) Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 5(9):1327–1333. https://doi.org/10.1002/elps.200305844
Chiarugi P, Cirri P, Marra F, Raugei G, Fiaschi T, Camici G (1998) The Src and signal transducers and activators of transcription pathways as specific targets for low molecular weight phosphotyrosine-protein phosphatase in platelet-derived growth factor signaling. J Biol Chem 273:6776–6785. https://doi.org/10.1074/jbc.273.12.6776
Chipuk JE, Green DR (2008) How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends in Cell Biol 18(4):157–164. https://doi.org/10.1016/j.tcb.2008.01.007
Czesnik D, Kuduz J, Schild D, Manzini I (2006) ATP activates both receptor and sustentacular supporting cells in the olfactory epithelium of Xenopus laevis tadpoles. Eur J Neurosci 23:119–128. https://doi.org/10.1111/j.1460-9568.2005.04533.x
Di Mauro S, Manes T, Hessle L, Kozlenkov A, Pizauro JM, Hoylaerts MF, Millan JL (2002) Kinetic characterization of hypophosphatasia mutations with physiological substrates. J Bone Miner Res 17(8):1383–1391. https://doi.org/10.1359/jbmr.2002.17.8.1383
Etkin W (1968) Metamorphosis: a problem in development biology. Appleton Century Crofts, New York
Fernley HN (1971) Mammalian alkaline phosphatases. In: Boyer PD (ed) The enzymes. Academic Press, New York, pp 417–447
Filburn CR, Vanable JW (1973) Acid phosphatase isozymes of Xenopus laevis tadpole tails: II. Changes in activity during tail regression. Arch Biochem Biophys 159:694–703. https://doi.org/10.1016/0003-9861(73)90509-2
Frezza C, Cipolat S, Scorrano L (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2(2):287–295. https://doi.org/10.1038/nprot.2006.478
Frisch SM, Francis H (1994) Disruption of epithelial cell–matrix interactions induces apoptosis. J Cell Biol 124:619–626. https://doi.org/10.1083/jcb.124.4.619
Fuchs Y, Steller H (2015) Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev 16(6):329–344. https://doi.org/10.1038/nrm3999
Gallego M, Virshup DM (2005) Protein serine/threonine phosphatases: life, death, and sleeping. Curr Opin Cell Biol 17:197–202. https://doi.org/10.1016/j.ceb.2005.01.002
Galluzzi L, Bravo-San Pedro JM, Vitale I et al (2015) Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ 22:58–73. https://doi.org/10.1038/cdd.2014.137
Garcia AF, Simão AM, Bolean M, Hoylaerts MF, Millán JL, Ciancaglini P, Costa-Filho AJ (2015) Effects of GPI-anchored TNAP on the dynamic structure of model membranes. Phys Chem Chem Phys 17(39):26295–26301. https://doi.org/10.1039/C5CP02377G
Gonçalves AM, Santos LFJ, Santana CC, Colosio RR, Pizauro JM (2015) Activity of tail phosphatases: a study during growth and metamorphosis of Lithobates catesbeianus. Copeia 103(3):634–638. https://doi.org/10.1643/OT-14-131
Gosner KLA (1960) Simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190. https://doi.org/10.2307/3890061
Hartree EF (1972) Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48:422–427. https://doi.org/10.1016/0003-2697(72)90094-2
Heroes E, Lesage B, Görnemann J, Beullens M, Meervelt LV, Bollen M (2012) The PP1 binding code: a molecular-lego strategy that governs specificity. FEBS J 280:584–595. https://doi.org/10.1111/j.1742-4658.2012.08547.x
Honkanen RE, Golden T (2002) Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr Med Chem 9:2055–2075. https://doi.org/10.2174/0929867023368836
Hoof CV, Goris J (2003) Phosphatases in apoptosis: to be or not to be, PP2A is in the heart of the question. Biochim Biophys Acta – Mol Cell Res 1640:97–104. https://doi.org/10.1016/S0167-4889(03)00029-6
Ishizuya-Oka A, Hasebe T, Shi Y (2009) Apoptosis in amphibian organs during metamorphosis. Apoptosis 15(3):350–364. https://doi.org/10.1007/s10495-009-0422-y
Janssens V, Login S, Goris J (2008) PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem Sci 33(3):113–121. https://doi.org/10.1016/j.tibs.2007.12.004
Kikuyama S, Kawamura K, Tanaka S, Yamamoto K (1993) Aspects of amphibian metamorphosis: hormonal control. Int Rev Cytol 145:105–148. https://doi.org/10.1016/S0074-7696(08)60426-X
Klumpp S, Krieglstein J (2002) Serine/threonine protein phosphatases in apoptosis. Curr Opin Pharmacol 2:458–462. https://doi.org/10.1016/s1471-4892(02)00176-5
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685. https://doi.org/10.1038/227680a0
Lin SS, Bassik MC, Suh H et al (2006) PP2A regulates BCL-2 phosphorylation and proteasome mediated degradation at the endoplasmic reticulum. J Biol Chem 281(32):23003–23012. https://doi.org/10.1074/jbc.M602648200
Mahapatra PK, Mohanty-Hejmadi M, Gagan BN (2001) Changes in oxidative stress parameters and acid phosphatase activity in the pre-regressing and regressing tail of indian jumping frog Polypedates maculatus (Anura, Rhacophoridae). Comp Biochem Phys C 130:281–288. https://doi.org/10.1016/s1532-0456(01)00238-1
Nakai Y, Nakajima K, Yaoita Y (2017) Mechanisms of tail resorption during anuran metamorphosis. Biomol Concepts 8(3–4):179–183. https://doi.org/10.1515/bmc-2017-0022
Nakajima K, Fujimoto K, Yaoita Y (2005) Programmed cell death during amphibian metamorphosis. Semin Cell Dev Biol 16:271–280. https://doi.org/10.1016/j.semcdb.2004.12.006
ORIGINLAB CORPORATION 2007 OriginPro Version 8. Northampton
Pfister N, Eickelberg O, Meiners S (2013) Proteasome function in lung fibrosis. Eur Respir J 42:P4886 Retrieved from https://erj.ersjournals.com/content/42/Suppl_57/P4886
Pizauro JM, Curti C, Ciancaglini P, Leone FA (1988) Kinetic properties of Triton X-100 solubilized bone matrix induced alkaline phosphatase. Cell Mol Biol 34:921–926. https://doi.org/10.1016/0305-0491(87)90413-5
Pizauro JM, Ciancaglini P, Leone FA (1995) Characterization of the phosphatidylinositol-specific phospholipase C-released form of rat osseous plate alkaline phosphatase and its possible significance on endochondral ossification. Mol Cell Biochem 22:121–129. https://doi.org/10.1007/BF01076074
Pizauro JM, Demenis MA, Ciancaglini P, Leone FA (1998) Kinetic characterization of a membrane-specific ATPase from rat osseous plate and its possible significance on endochondral ossification. Biochim Biophys Acta 1368:108–114. https://doi.org/10.1016/s0005-2736(97)00174-0
Raugei G, Ramponi G, Chiarugi P (2002) Low molecular weight protein tyrosine phosphatases: small, but smart. Cell Mol Life Sci 59:941–949. https://doi.org/10.1007/s00018-002-8481-z
Robinson H (1970) Acid phosphatase in the tail of Xenopus laevis during development and metamorphosis. J Exp Zool 173:215–224. https://doi.org/10.1002/jez.1401730209
Robinson H (1972) An electrophoretic and biochemical analysis of acid phosphatase in the tail of Xenopus laevis during development and metamorphosis. J Exp Zool 180:127–140. https://doi.org/10.1002/jez.1401800111
Santos LFJ, Oliveira-Bahia VRL, Nakaghi LSO, De Stefani MV, Gonçalves AM, Pizauro JM (2016) Ontogeny of the digestive enzymes of tadpoles of Lithobates catesbeianus. Copeia 104(4):838–842. https://doi.org/10.1643/CG-16-432
Segel IH (1975) Enzymes kinetics: behavior and analysis of rapid equilibrium and steady-state enzymes systems. Wiley, New York
Sesana S, Re F, Bulbarelli A, Salerno D, Cazzaniga E, Masserini M (2008) Membrane features and activity of GPI-anchored enzymes: alkaline phosphatase reconstituted in model membranes. Biochemistry 47(19):5433–5440. https://doi.org/10.1021/bi800005s
Shevchenko A, Tomas H, Havli J, Olsen JV, Mann M (2007) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860. https://doi.org/10.1038/nprot.2006.468
Siddiqui RA, Jenski LJ, Neff K, Harvey K, Kovacs RJ, Stillwell W (2001) Docosahexaenoic acid induces apoptosis in Jurkat cells by a protein phosphatase-mediated process. Biochim Biophy Acta 1499:265–275. https://doi.org/10.1016/S0167-4889(00)00128-2
Sun H, Wang Y (2012) Novel Ser/Thr protein phosphatases in cell death regulation. Physiology 27:43–52. https://doi.org/10.1152/physiol.00034.2011
Tait SWG, Green DR (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev 11:621–632. https://doi.org/10.1038/nrm2952
Tata JR (1966) Requirement for RNA and protein synthesis for induced regression of the tadpole tail in organ culture. Dev Biol 13:77–94. https://doi.org/10.1016/0012-1606(66)90050-9
Tonks NK (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev 7:833–846.https://doi.org/10.1038/nrm2039
Virshup DM, Shenolikar S (2009) From promiscuity to precision: protein phosphatases get a makeover. Mol Cell 33:537–545. https://doi.org/10.1016/j.molcel.2009.02.015
Zhang Z (2002) Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Annu Rev Pharmacol Toxicol 42:209–234. https://doi.org/10.1146/annurev.pharmtox.42.083001.144616
Acknowledgments
We are grateful to all the members of LEIA (Laboratório de Enzimologia e Imunoquímica Aplicadas).
Code availability
Not applicable.
Funding
This work was supported by CAPES and FAPESP.
Author information
Authors and Affiliations
Contributions
AMG, CCS, RRC and LFJS and classified the animals, executed the experiments and participated on results discussion. AMG performed the statistical analyses. TSB performed mass spectrometry analyses and result interpretation. AMG and JMPJ conceived of the study and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The authors state that all procedures were performed with the approval of the Ethics Committee on Animal Use of the University (Protocol No. 010537/11).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable.
Conflicts of interest/competing interests
The authors declare that there is not any conflict of interest within this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Fig. 1
Scheme for obtaining soluble and membrane proteins (PNG 65 kb)
Supplementary Fig. 2
Scheme of mitochondrial isolation from the tail of L. catesbeianus tadpole (PNG 30 kb)
Supplementary Fig. 3
Effect of pNPP concentration on p-nitrophenylphosphatase activity of the phosphatases present in the tail of stage 44 L. catesbeianus tadpoles. A- Alkaline phosphatase solubilized with PIPLC. B- Membrane-bound acid phosphatase. C- Soluble acid phosphatase. (PNG 41 kb)
Supplementary Fig. 4
Apparent optimum pH of alkaline phosphatase activity present in the tail membrane fraction of stage 44 L. catesbeianus tadpoles. A- pNPP 1 mM. B- pNPP 0.1 mM (PNG 311 kb)
ESM 5
(DOCX 15 kb)
ESM 6
(DOCX 13 kb)
ESM 7
(DOCX 13 kb)
ESM 8
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Gonçalves, A.M., Santana, C.C., Santos, L.F.J.D. et al. Identification and characterization of acid and alkaline phosphatases and protein phosphatases in L. catesbeianus tail during metamorphosis. Biologia 76, 3521–3531 (2021). https://doi.org/10.1007/s11756-021-00877-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00877-9