Abstract
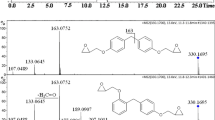
The volatile compound trans-4,5-epoxy-(E)-2-decenal (1) was synthesized in two steps with good overall yields. The newly developed method is based on trans-epoxidation of (F)-2-octenal with alkaline hydrogen peroxide followed by a Wittig-type chain elongation with the ylide formylmethylene triphenylphosphorane. For the synthesis of [4,5-2H2]-trans-4,5-epoxy-(E)-2-decenal (d-1), [2,3-2H2]-(E)-2-octenal was prepared by reduction of 2-octyn-1-ol with lithium aluminum deuteride and subsequent oxidation of [2,3-2H2]-(E)-2-octen-1-ol with manganese oxide. Compound d-1 was used as internal standard for the quantification of 1 by isotope dilution assay. Among various mass spectrometry (MS) ionization techniques tested, negative chemical ionization with ammonia as reagent gas gave best results with respect to both sensitivity and selectivity. The detection limit was found to be at about 1 pg of the analyte introduced into the gas chromatography-MS system.
Similar content being viewed by others
Abbreviations
- CC:
-
column chromatography
- COSY:
-
homonuclear correlation spectroscopy
- EI:
-
electron ionization
- GC:
-
gas chromatography
- HETCOR:
-
heteronuclear correlation spectra
- IDA:
-
isotope dilution assay
- MS:
-
mass spectrometry
- NCI:
-
negative chemical ionization
- NMR:
-
nuclear magnetic resonance
- NOE:
-
nuclear Overhauser effect
- PC:
-
phosphatidylcholine
- PCI:
-
positive chemical ionization
- PE:
-
phosphatidylethanolamine
- RI:
-
retention index
- SIM:
-
selected ion monitoring
- THF:
-
tetrahydrofuran
References
Selke, E., Rohwedder, W.K., and Dutton, H.J. (1980) Volatile Components from Trilinolein Heated in Air, J. Am. Oil Chem. Soc. 57, 25–30.
Gardner, H.W., and Selke, E. (1984) Volatiles from Thermal Decomposition of Isomeric Methyl (12S,13S)-(E)-12,13-epoxy-9-hydroperoxy-10-octadecenoates, Lipids 19, 375–380.
Gassenmeier, K., and Schieberle, P. (1994) Formation of the Intense Flavor Compound trans-4,5-Epoxy-(E)-2-decenal in Thermally Treated Fats, J. Am. Oil Chem. Soc. 71, 1315–1319.
Guth, H., and Grosch, W. (1990) Deterioration of Soya-Bean Oil: Quantification of Primary Flavour Compounds Using a Stable Isotope Dilution Assay, Lebensm. Wiss. Technol. 23, 513–522.
Grosch, W. (1993) Detection of Potent Odorants in Foods by Aroma Extract Dilution Analysis, Trends Food Sci. Technol. 4, 68–73.
Schieberle, P., and Grosch, W. (1991) Potent Odorants of the Wheat Bread Crumb. Differences to the Crust and Effect of a Longer Dough Fermentation, Z. Lebensm. Unters. Forsch. 192, 130–135.
Guth, H., and Grosch, W. (1990) Comparison of Stored Soya-bean and Rapeseed Oils by Aroma Extract Dilution Analysis, Lebensm. Wiss. Technol. 23, 59–65.
Konopka, U.C., and Grosch, W. (1991) Potent Odorants Causing the Warmed-Over Flavour in Boiled Beef, Z. Lebensm. Unters. Forsch. 193, 123–125.
Kerler, J., and Grosch, W. (1997) Character Impact Odorants of Boiled Chicken: Changes During Refrigerated Storage and Reheating, Z. Lebensm. Unters. Forsch. 205, 232–238.
Konopka, U.C., Guth, H., and Grosch, W. (1995) Potent Odorants Formed by Lipid Peroxidation as Indicators of the Warmed-over Flavour (WOF) of Cooked Meat, Z. Lebensm. Unters. Forsch. 201, 339–343.
Jürgens, G., Lang, J., and Esterbauer, H. (1986) Modification of Human Low-Density Lipoprotein by the Lipid Peroxidation Product 4-Hydroxynonenal, Biochim. Biophys. Acta 875, 103–114.
Zamora, R., and Hidalgo, F.J. (1995) Linoleic Acid Oxidation in the Presence of Amino Compounds Produces Pyrrols by Carbonyl Amine Reactions, Biochim. Biophys. Acta 1258, 319–327.
Grein, B., Huffer, M., Scheller, G., and Schreier, P. (1993) 4-Hydroxy-2-alkenals and Other Products Formed by Water-Mediated Oxidative Decomposition of α,β-Unsaturated Aldehydes, J. Agric. Food Chem. 41, 2385–2390.
Benedetti, A., Comporti, M., Fulceri, R., and Esterbauer, H. (1984) Cytotoxic Aldehydes Originating from the Peroxidation of Liver Microsomal Lipids. Identification of 4,5-Dihydroxydecenal, Biochim. Biophys. Acta 792, 172–181.
Zamora, R., and Hidalgo, F.J. (1994) Modification of Lysine Amino Acids by the Lipid Peroxidation Product 4,5(E)-Epoxy-2(E)-heptenal, Lipids 29, 243–249.
Hidalgo, F.J., and Zamora, R. (1993) Fluorescent Pyrrole Products from Carbonyl-Amine Reactions, J. Biol. Chem. 22, 16190–16197.
Chang, S., Lee, N.H., and Jacobsen, E.N. (1993) Regio- and Enantioselective Catalytic Epoxidation of Conjugated Polyenes. Formal Synthesis of LTA4 Methyl Ester, J. Org. Chem. 58, 6939–6941.
Guth, H., and Grosch, W. (1993) Quantitation of Potent Odorants of Virgin Olive Oil by Stable-Isotope Dilution Assays, J. Am. Oil Chem. Soc. 70, 513–518.
Schieberle, P., and Grosch, W. (1987) Quantitative Analysis of Aroma Compounds in Wheat and Rye Bread Crusts Using Stable Isotope Dilution Assay, J. Agric. Food. Chem. 35, 252–257.
Sen, A., Laskawy, G., Schieberle, P., and Grosch, W. (1991) Quantitative Determination of β-damascenone in Foods Using a Stable Isotope Dilution Assay, J. Agric. Food Chem. 39, 757–759.
van den Dool, H., and Kratz, P. (1963) A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography, J. Chromatogr. 24, 463–471.
Lin, J., Welti, D.H., Arce Vera, F., Fay, L.B., and Blank, I. (1999) Synthesis of Deuterated Volatile Lipid Degradation Products to Be Used as Internal Standards in Isotope Dilution Assays. 1. Aldehydes, J. Agric. Food Chem. 47, 2813–2821.
Attenburrow, J., Cameron, A.F.B., Chapman, J.H., Evans, R.M., Hems, B.A., Jansen, A.B.A., and Walker, T. (1952) A Synthesis of Vitamin A from Cyclohexanone, J. Chem. Soc., 1094–1111.
Felix, D., Winter, C., and Eschenmoser, A. (1988) Fragmentation of α,β-Epoxyketones to Acetylenic Aldehydes and Ketones: Preparation of 2,3-Epoxy-cyclohexanone and Its Fragmentation to 5-Hexynal, Org. Synthesis 6, 679–682.
Gleason, J.G., Bryan, D.B., and Kinzig, C.M. (1980) Convergent Synthesis of Leukotriene A Methyl Ester, Tetrahedron Lett. 21, 1129–1132.
Pritzkow, W., Radeglia, R., and Schmidt-Renner, W. (1979) Studies on the Autoxidation of the Isomeric n-Octenes, J. Prakt. Chem. 321(5), 813–826 [in German; Chem. Abstr. 92 (1980), 75520c].
Swoboda, P.A.T., and Peers, K.E. (1978) trans-4,5-Epoxyhept-trans-2-enal. The Major Volatile Compound Formed by the Copper and α-Tocopherol Induced Oxidation of Butterfat, J. Sci. Food Agric. 29, 803–807.
Pierre, J.L., Chautemps, P., Arnaud, P., and Gey, C. (1968) Nuclear Magnetic Resonance of Small Cyclic Compounds. VI. α-Epoxy Alcohols, Chim. Anal. 50, 494–500 [in French; Chem. Abstr. 70 (1969) 24505q].
Wolfe, R.R. (1984) Tracers in Metabolic Research: Radioisotope and Stable Isotope/Mass Spectrometry Methods, pp. 189–206, alan R. Liss Inc., New York.
Staempfli, A.A., Blank, I., Fumeaux, R., and Fay, L.B. (1994) Study on the Decomposition of the Amadori Compound N-(1-deoxy-d-fructos-1-yl)-glycine in Model Systems: Quantification by Fast Atom Bombardment Tandem Mass Spectrometry, Biol. Mass Spectrom. 23, 642–646.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lin, J., Fay, L.B., Welti, D.H. et al. Synthesis of trans-4,5-epoxy-(E)-2-decenal and its deuterated analog used for the development of a sensitive and selective quantification method based on isotope dilution assay with negative chemical ionization. Lipids 34, 1117–1126 (1999). https://doi.org/10.1007/s11745-999-0463-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-999-0463-8