Abstract
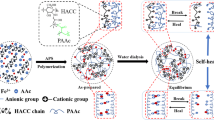
Autonomous self-healing hydrogels were achieved through a dynamic combination of hydrogen bonding and ferric ion (Fe3+) migration. N,N′-methylenebis (acrylamide) (MBA), a cross-linking agent, was added in this study. Poly(acrylic acid) (PAA)/Fe3+ and PAA–MBA/Fe3+ hydrogels were prepared by introducing Fe3+ into the PAA hydrogel network. The ionic bonds were formed between Fe3+ ions and carboxyl groups. The microstructure, mechanical properties, and composition of hydrogels were characterized by field emission scanning electron microscopy and Fourier transform infrared spectroscopy. The experimental results showed that PAA/Fe3+ and PAA–MBA/Fe3+ hydrogels healed themselves without external stimuli. The PAA/Fe3+ hydrogel exhibited good mechanical properties, i.e., the tensile strength of 50 kPa, the breaking elongation of 750%, and the self-healing efficiency of 82%. Meanwhile, the PAA–MBA/Fe3+ hydrogel had a tensile strength of 120 kPa. These fabricated hydrogels are biocompatible, which may have promising applications in cartilage tissue engineering.
Similar content being viewed by others
References
Francis Suh J K, Matthew H W T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials, 2000, 21(24): 2589–2598
Martel-Pelletier J, Boileau C, Pelletier J P, et al. Cartilage in normal and osteoarthritis conditions. Best Practice & Research. Clinical Rheumatology, 2008, 22(2): 351–384
Hunter W. Of the structure and disease of articulating cartilages. 1743. Clinical Orthopaedics and Related Research, 1995, (317): 3–6
Buckwalter J A, Lane N E. Athletics and osteoarthritis. The American Journal of Sports Medicine, 1997, 25(6): 873–881
Martin J A, Buckwalter J A. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. The Iowa Orthopaedic Journal, 2001, 21: 1–7
Martin J A, Buckwalter J A. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology, 2002, 3(5): 257–264
de Windt T S, Vonk L A, Brittberg M, et al. Treatment and prevention of (early) osteoarthritis using articular cartilage repair — fact or fiction? A systematic review Cartilage, 2013, 4(3 Suppl): 5S–12S
Chang C-H, Lin F-H, Kuo T-F, et al. Cartilage tissue engineering. Biomedical Engineering: Applications, Basis and Communications, 2005, 17(2): 61–71
Solchaga L A, Goldberg V M, Caplan A I. Cartilage regeneration using principles of tissue engineering. Clinical Orthopaedics and Related Research, 2001, 391(Suppl): S161–S170
Buckwalter J A, Mankin H J. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instructional Course Lectures, 1998, 47: 487–504
Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee, 2007, 14(3): 177–182
Athanasiou K A, Shah A R, Hernandez R J, et al. Basic science of articular cartilage repair. Clinics in Sports Medicine, 2001, 20(2): 223–247
Hayes D W Jr, Brower R L, John K J. Articular cartilage. Anatomy, injury, and repair. Clinics in Podiatric Medicine and Surgery, 2001, 18(1): 35–53
Hunziker E B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and Cartilage, 2002, 10(6): 432–463
Zheng Q, Ma Z, Gong S. Multi-stimuli-responsive self-healing metallo–supramolecular polymer nanocomposites. Journal of Materials Chemistry A: Materials for Energy and Sustainability, 2016, 4(9): 3324–3334
Song M M, Wang Y M, Liang X Y, et al. Functional materials with self-healing properties: a review. Soft Matter, 2019, 15(33): 6615–6625
Beddoes C M, Whitehouse M R, Briscoe W H, et al. Hydrogels as a replacement material for damaged articular hyaline cartilage. Materials, 2016, 9(6): 443
Li L, Yu F, Zheng L, et al. Natural hydrogels for cartilage regeneration: modification, preparation and application. Journal of Orthopaedic Translation, 2019, 17: 26–41
Liu J, Qu S, Suo Z, et al. Functional hydrogel coatings. National Science Review, 2021, 8(2): nwaa254
Calvert P. Hydrogels for soft machines. Advanced Materials, 2009, 21(7): 743–756
Taylor D L, Panhuis M I H. Self-healing hydrogels. Advanced Materials, 2016, 28(41): 9060–9093
Hillewaere X K D, Du Prez F E. Fifteen chemistries for autonomous external self-healing polymers and composites. Progress in Polymer Science, 2015, 49–50: 121–153
Bakarich S E, Gorkin R, Panhuis M I H, et al. Three-dimensional printing fiber reinforced hydrogel composites. ACS Applied Materials & Interfaces, 2014, 6(18): 15998–16006
Yu F, Cao X, Du J, et al. Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining die is alder click reaction and acylhydrazone bond. ACS Applied Materials & Interfaces, 2015, 7(43): 24023–24031
Yang L, Wang Z, Fei G, et al. Polydopamine particles reinforced poly(vinyl alcohol) hydrogel with NIR light triggered shape memory and self-healing capability. Macromolecular Rapid Communications, 2017, 38(23): 1700421
Yang W J, Tao X, Zhao T, et al. Antifouling and antibacterial hydrogel coatings with self-healing properties based on a dynamic disulfide exchange reaction. Polymer Chemistry, 2015, 6(39): 7027–7035
Tie J, Liu H, Lv J, et al. Multi-responsive, self-healing and adhesive PVA based hydrogels induced by the ultrafast complexation of Fe3+ ions. Soft Matter, 2019, 15(37): 7404–7411
Zhang H, Xia H, Zhao Y. Poly(vinyl alcohol) hydrogel can autonomously self-heal. ACS Macro Letters, 2012, 1(11): 1233–1236
Okay O. In: Seiffert S, ed. Supramolecular Polymer Networks and Gels. Springer, 2015
Deng Y, Huang M, Sun D, et al. Dual physically cross-linked κ-carrageenan-based double network hydrogels with superior self-healing performance for biomedical application. ACS Applied Materials & Interfaces, 2018, 10(43): 37544–37554
Zhou H, Xu G, Li J, et al. Preparation and self-healing behaviors of poly(acrylic acid)/cerium ions double network hydrogels. Macromolecular Research, 2015, 23(12): 1098–1102
Dalei G, Das S. Polyacrylic acid-based drug delivery systems: a comprehensive review on the state-of-art. Journal of Drug Delivery Science and Technology, 2022, 78: 103988
Peng F, Li G, Liu X, et al. Redox-responsive gel–sol/sol–gel transition in poly(acrylic acid) aqueous solution containing Fe(III) ions switched by light. Journal of the American Chemical Society, 2008, 130(48): 16166–16167
Anjum S, Gurave P, Badiger M V, et al. Design and development of trivalent aluminum ions induced self healing polyacrylic acid novel hydrogels. Polymer, 2017, 126: 196–205
Gosset M, Berenbaum F, Thirion S, et al. Primary culture and phenotyping of murine chondrocytes. Nature Protocols, 2008, 3(8): 1253–1260
Gumbiner B M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell, 1996, 84(3): 345–357
Parker K K, Brock A L, Brangwynne C, et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB Journal, 2002, 16(10): 1195–1204
Acknowledgements
This work was supported by the General Project of Natural Science of Shanxi Provincial Basic Research Program (Grant No. 202203021211125) and the National Natural Science Foundation of China (Grant No. 11802197).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interests No potential conflict of interest was reported by the authors.
Rights and permissions
About this article
Cite this article
Kang, M., Cheng, Y., Hu, Y. et al. Self-healing poly(acrylic acid) hydrogels fabricated by hydrogen bonding and Fe3+ ion cross-linking for cartilage tissue engineering. Front. Mater. Sci. 17, 230655 (2023). https://doi.org/10.1007/s11706-023-0655-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11706-023-0655-7