Abstract
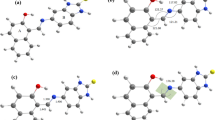
The studies on molecular interactions of drug candidates with HSA have considerably contributed to the understanding of primary biological evaluation of potential drug. In this context, Schiff base, (E)-2-((2-hydroxy-3-methoxybenzylidene)amino)-6H-benzo[c]chromen-6-one (1) derived from o-vanillin and 6-amino-3,4-benzocoumarin was synthesized and characterized by elemental analysis, UV–Vis, IR, NMR, and ESI–MS. The HSA interaction studies of compound 1 was carried out employing various biophysical techniques, viz., UV–Vis titration, fluorescence quenching experiments, and CD measurements. The compound 1 displayed non-covalent hydrophobic mode of binding (Kb = 3.4 × 103 M−1). The fluorescence spectroscopic results revealed that compound 1 exhibited strong capability to quench the intrinsic fluorescence of HSA at Trp214 site (Ksv = 4.1 × 103 M−1). Also ADME and Lipinski’s drug-likeness predicted favorable physicochemical properties for oral bioavailability. To ascertain the specific binding to HSA, molecular docking was performed deciphering that electrostatic steady-state interactions have key role in binding of compound 1 to HSA with ΔG of −9.21 kcal mol−1. Moreover, DFT calculations were conducted to obtain electronic and structural properties of compound 1. The theoretical calculations and the experimental data are in well agreement, and hence, the findings suggest that compound 1 might exhibit a potent role in the biomedical and pharmaceutical application.











Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption, dispensation, metabolism, and excretion
- Arg:
-
Arginine
- BBB:
-
Blood brain barrier
- CD:
-
Circular dichroism
- Cys:
-
Cysteine
- DFT:
-
Density functional theory
- ESI:
-
Electron spray ionization
- FMO:
-
Frontier molecular orbital
- GI:
-
Gastrointestinal
- Gln:
-
Glutamine
- Glu:
-
Glutamic acid
- His:
-
Histidine
- HIV:
-
Human immune deficiency virus
- HOMO:
-
Highest occupied molecular orbital
- HSA:
-
Human serum albumin
- IR:
-
Infrared
- Leu:
-
Leucine
- LUMO:
-
Lowest unoccupied molecular orbital
- Lys:
-
Lysine
- MS:
-
Mass spectrometry
- NMR:
-
Nuclear magnetic resonance
- Phe:
-
Phenylalanine
- Ser:
-
Serine
- TPSA:
-
Topological polar surface area
- Trp:
-
Tryptophan
- Tyr:
-
Tyrosine
- UV–Vis:
-
Ultraviolet–visible
References
Abdel-Mohsen HT et al (2020) Synthesis, crystal structure, and ADME prediction studies of novel imidazopyrimidines as antibacterial and cytotoxic agents. Arch Pharm. https://doi.org/10.1002/ardp.201900271
Agrawal R et al (2019) Elucidating the binding propensity of naphthyl hydroxamic acid to human serum albumin (HSA): multi-spectroscopic and molecular modeling approach. J Mol Struct 1184:1–11. https://doi.org/10.1016/j.molstruc.2019.01.067
Agudelo D et al (2012) Probing the binding sites of antibiotic drugs doxorubicin and N-(trifluoroacetyl) doxorubicin with human and bovine serum albumins sem, DS, editor. PLoS ONE 7:e43814. https://doi.org/10.1371/journal.pone.0043814
Ajmal MR et al (2017) Interaction of anticancer drug clofarabine with human serum albumin and human α-1 acid glycoprotein. Spectroscopic and molecular docking approach. J Pharm Biomed Anal 135:106–115. https://doi.org/10.1016/j.jpba.2016.12.001
Alanazi AM et al (2018) Spectroscopic and molecular docking studies of the binding of the angiotensin II receptor blockers (ARBs) azilsartan, eprosartan and olmesartan to bovine serum albumin. J Lumin 203:616–628. https://doi.org/10.1016/j.jlumin.2018.06.085
Alyar S et al (2019) Synthesis, spectroscopic characterizations, enzyme inhibition, molecular docking study and DFT calculations of new Schiff bases of sulfa drugs. J Mol Struct 1185:416–424. https://doi.org/10.1016/j.molstruc.2019.03.002
Baek NI, Ahn EM, Kim HY, Park YD (2000) Furanocoumarins from the root of Angelica dahurica. Arch Pharm Res 23:467–470. https://doi.org/10.1007/BF02976574
Bagheri M, Fatemi MH (2018) Fluorescence spectroscopy, molecular docking and molecular dynamic simulation studies of HSA-Aflatoxin B1 and G1 interactions. J Lumin 202:345–353. https://doi.org/10.1016/j.jlumin.2018.05.066
Balakrishnan G et al (2019) Synthesis, photophysics and the binding studies of rhenium(I) diimine surfactant complexes with serum albumins: a spectroscopic and docking study approach. J Lumin 205:51–60. https://doi.org/10.1016/j.jlumin.2018.08.078
Bansal Y, Sethi P, Bansal G (2013) Coumarin: A potential nucleus for anti-inflammatory molecules. Med Chem Res 22:3049–3060. https://doi.org/10.1007/s00044-012-0321-6
Barik A, Mishra B, Kunwar A, Indira PK (2007) Interaction of curcumin with human serum albumin: thermodynamic properties, fluorescence energy transfer and denaturation effects. Chem Phys Lett 436:239–243. https://doi.org/10.1016/j.cplett.2007.01.006
Chaves O et al (2015) Fluorescence and docking studies of the interaction between human serum albumin and pheophytin. Molecules 20:19526–19539. https://doi.org/10.3390/molecules201019526
Chaves OA et al (2019) In vitro study of the interaction between HSA and 4-bromoindolylchalcone, a potent human MAO-B inhibitor: spectroscopic and molecular modeling studies. Chem Select 4:1007–1014. https://doi.org/10.1002/slct.201802665
Chaves OA et al (2018) Synthesis, tyrosinase inhibition and transportation behavior of novel β-enamino thiosemicarbazide derivatives by human serum albumin. J Mol Liq 254:280–290. https://doi.org/10.1016/j.molliq.2018.01.083
Cheng YH, Zhao X, Song KS, Liu L, Guo QX (2002) Remote substituent effects on bond dissociation energies of para-substituted aromatic silanes. J Org Chem 67:6638–6645. https://doi.org/10.1021/jo020085h
Cheng W et al (2014) Identification of novel 4-anilinoquinazoline derivatives as potent EGFR inhibitors both under normoxia and hypoxia. Bioorgan Med Chem 22:6796–6805. https://doi.org/10.1016/j.bmc.2014.10.038
Cozza G et al (2011) Urolithin as a converging scaffold linking ellagic acid and coumarin analogues: design of potent protein kinase CK2 inhibitors. ChemMedChem 6:2273–2286. https://doi.org/10.1002/cmdc.201100338
Daina A, Michielin O, Zoete V (2017) SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717. https://doi.org/10.1038/srep42717
De A, Ray HP, Jain P, Kaur H, Singh N (2020) Synthesis, characterization, molecular docking and DNA cleavage study of transition metal complexes of o-vanillin and glycine derived Schiff base ligand. J Mol Struct 1199:126901. https://doi.org/10.1016/j.molstruc.2019.126901
Frisch MJ et al (2009) ‘Gaussian 09; Gaussian. Inc’, Wallingford, CT, p 32
Fukui K (1982) Role of frontier orbitals in chemical reactions. Science 218:747–754. https://doi.org/10.1126/science.218.4574.747
González-Barrio R, Truchado P, Ito H, Espín JC, Tomás-Barberán FA (2011) UV and MS identification of urolithins and nasutins, the bioavailable metabolites of ellagitannins and ellagic acid in different mammals. J Agric Food Chem 59:1152–1162. https://doi.org/10.1021/jf103894m
Gupta RK et al (2013) DNA/protein binding, molecular docking, and in vitro anticancer activity of some thioether-dipyrrinato complexes. Inorg Chem 52:13984–13996. https://doi.org/10.1021/ic401662d
Husain A et al (2020) Synthesis, in vitro cytotoxicity, ADME and molecular docking studies of benzimidazole‐bearing furanone derivatives. J Chin Chem Soc. https://doi.org/10.1002/jccs.202000130
Husseinzadeh N, Davenport SM (2014) Role of toll-like receptors in cervical, endometrial and ovarian cancers: a review. Gynecol Oncol 135:359–363. https://doi.org/10.1016/j.ygyno.2014.08.013
Jafari F et al (2018) Experimental and computational studies on the binding of diazinon to human serum albumin. J Biomol Struct Dyn 36:1490–1510. https://doi.org/10.1080/07391102.2017.1329096
Kiss AK, Granica S, Stolarczyk M, Melzig MF (2012) Epigenetic modulation of mechanisms involved in inflammation: influence of selected polyphenolic substances on histone acetylation state. Food Chem 131:1015–1020. https://doi.org/10.1016/j.foodchem.2011.09.109
Kiyooka SI, Kaneno D, Fujiyama R (2013) Parr’s index to describe both electrophilicity and nucleophilicity. Tetrahedron Lett 54:339–342. https://doi.org/10.1016/j.tetlet.2012.11.039
Koley Seth B et al (2016) Structure dependent hydrophobic and hydrophilic interactions between nickel(II) Schiff base complexes and serum albumins: spectroscopic and docking studies. J Lumin 171:85–97. https://doi.org/10.1016/j.jlumin.2015.11.009
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, Boston. https://doi.org/10.1007/978-0-387-46312-4
Lakowicz JR, Weber G (1973) Quenching of fluorescence by oxygen. A probe for structural fluctuations in macromolecules. Biochemistry. https://doi.org/10.1021/bi00745a020
Li JWH, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325:161–165. https://doi.org/10.1126/science.1168243
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25. https://doi.org/10.1016/S0169-409X(96)00423-1
Lou Y-Y, Zhou K-L, Shi J-H, Pan D-Q (2017) Characterizing the binding interaction of fungicide boscalid with bovine serum albumin (BSA): a spectroscopic study in combination with molecular docking approach. J Photochem Photobiol B Biol 173:589–597. https://doi.org/10.1016/j.jphotobiol.2017.06.037
Hassan M, Azzazy E, Christenson RH (1997) All about albumin: biochemistry, genetics, and medical applications. Theodore Peters, Jr. San Diego, CA: Academic Press, 1996, 432 pp, $85.00. ISBN 0-12-552110-3. Clin Chem 43:2014a–2015. https://doi.org/10.1093/clinchem/43.10.2014a
Manjushree M, Revanasiddappa HD (2020) Evaluation of binding mode between anticancer drug etoposide and human serum albumin by numerous spectrometric techniques and molecular docking. Chem Phys 530:110593. https://doi.org/10.1016/j.chemphys.2019.110593
Mahendiran D et al (2018) Bis(thiosemicarbazone)copper(I) complexes as prospective therapeutic agents: interaction with DNA/BSA molecules, and in vitro and in vivo anti-proliferative activities. ChemistrySelect 3:7100–7111. https://doi.org/10.1002/slct.201800934
Majumder A, Rosair GM, Mallick A, Chattopadhyay N, Mitra S (2006) Synthesis, structures and fluorescence of nickel, zinc and cadmium complexes with the N, N, O-tridentate Schiff base N-2-pyridylmethylidene-2-hydroxy-phenylamine. Polyhedron. https://doi.org/10.1016/j.poly.2005.11.029
Marton A et al (2016) Vanillin analogues o-vanillin and 2,4,6-trihydroxybenzaldehyde inhibit NFκB activation and suppress growth of A375 human melanoma. Anticancer Res 36:5743–5750. https://doi.org/10.21873/anticanres.11157
Mohammadnia F, Fatemi MH, Taghizadeh SM (2020) Study on the interaction of anti-inflammatory drugs with human serum albumin using molecular docking, quantitative structure–activity relationship, and fluorescence spectroscopy. Luminescence 35:266–273. https://doi.org/10.1002/bio.3723
Mondal M, Pragna Lakshmi T, Krishna R, Sakthivel N (2017) Molecular interaction between human serum albumin (HSA) and phloroglucinol derivative that shows selective anti-proliferative potential. J Lumin 192:990–998. https://doi.org/10.1016/j.jlumin.2017.08.007
Morris GM et al (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. https://doi.org/10.1002/jcc.21256
Nunhart P et al (2020) Antimicrobial activity and DNA/HSA interaction of fluorinated 3,6,9-trisubstituted acridines. Chem Pap 74:2327–2337. https://doi.org/10.1007/s11696-020-01079-4
Oh H et al (2002) Hepatoprotective and free radical scavenging activities of prenylflavonoids, coumarin, and stilbene from Morus alba. Planta Med 68:932–934. https://doi.org/10.1055/s-2002-34930
Otávio C et al (2018) Multi-spectroscopic and theoretical analysis on the interaction between human serum albumin and a capsaicin derivative—RPF101. Biomolecules 8:78. https://doi.org/10.3390/biom8030078
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Parthasarathi R, Subramanian V, Roy DR, Chattaraj PK (2004) Electrophilicity index as a possible descriptor of biological activity. Bioorg Med Chem 12:5533–5543. https://doi.org/10.1016/j.bmc.2004.08.013
Parveen S (2021) In silico drug repurposing of fda-approved artemisinins as potent chemotherapeutics targeting BCL-2, CDK-6 & VEGFR-2: density functional exploration and molecular docking study. Biointerface Res Appl Chem 11:9604–9618. https://doi.org/10.33263/BRIAC112.96049618
Parveen S, Arjmand F et al (2020a) Molecular docking, DFT and antimicrobial studies of Cu(II) complex as topoisomerase I inhibitor. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1743365
Parveen S, Alnoman RB, Hagar M, Ahmed HA, Knight JG (2020b) Synthesis, molecular docking, and DFT calculation of a half-strapped BODIPY as potential EGFR inhibitor**. ChemistrySelect 5:13163–13173. https://doi.org/10.1002/slct.202003621
Pawar SK, Kalalbandi VKA, Jaldappagari S (2018) Interaction of indole derivative with human serum albumin: a combined spectroscopic and molecular dynamics study. ChemistrySelect 3:12080–12088. https://doi.org/10.1002/slct.201802466
Pearson RG (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci 83:8440–8441. https://doi.org/10.1073/pnas.83.22.8440
Petitpas I, Bhattacharya AA, Twine S, East M, Curry S (2001) Crystal structure analysis of warfarin binding to human serum albumin. J Biol Chem 276:22804–22809. https://doi.org/10.1074/jbc.M100575200
Poureshghi F, Ghandforoushan P, Safarnejad A, Soltani S (2017) Interaction of an antiepileptic drug, lamotrigine with human serum albumin (HSA): application of spectroscopic techniques and molecular modeling methods. J Photochem Photobiol B Biol 166:187–192. https://doi.org/10.1016/j.jphotobiol.2016.09.046
Rabbani G et al (2017) Biophysical study on the interaction between eperisone hydrochloride and human serum albumin using spectroscopic, calorimetric, and molecular docking analyses. Mol Pharm 14:1656–1665. https://doi.org/10.1021/acs.molpharmaceut.6b01124
Rosselli S et al (2009) The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol. Nat Prod Commun 4:1701–1706. https://doi.org/10.1177/1934578x0900401219
Sashidhara KV, Kumar A, Kumar M, Sarkar J, Sinha S (2010) Synthesis and in vitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents. Bioorg Med Chem Lett 20:7205–7211. https://doi.org/10.1016/j.bmcl.2010.10.116
Sashidhara KV et al (2011) Discovery and synthesis of novel 3-phenylcoumarin derivatives as antidepressant agents. Bioorg Med Chem Lett 21:1937–1941. https://doi.org/10.1016/j.bmcl.2011.02.040
Setena SM, Shah NM (1945) The chemistry of coumarins. Chem Rev 36:1–62. https://doi.org/10.1021/cr60113a001
Sevilla P, Rivas JM, García-Blanco F, García-Ramos JV, Sánchez-Cortés S (2007) Identification of the antitumoral drug emodin binding sites in bovine serum albumin by spectroscopic methods. Biochim Biophys Acta 1774:1359–1369. https://doi.org/10.1016/j.bbapap.2007.07.022
Shahabadi N, Zendehcheshm S (2020) Evaluation of ct-DNA and HSA binding propensity of antibacterial drug chloroxine: multi-spectroscopic analysis, atomic force microscopy and docking simulation. Spectrochim Acta Part A Mol Biomol Spectrosc 230:118042. https://doi.org/10.1016/j.saa.2020.118042
Shahabadi N, Fili SM, Kheirdoosh F (2013) Study on the interaction of the drug mesalamine with calf thymus DNA using molecular docking and spectroscopic techniques. J Photochem Photobiol B Biol 128:20–26. https://doi.org/10.1016/j.jphotobiol.2013.08.005
Sherafati M et al (2020) Design, synthesis and biological evaluation of novel phthalimide-Schiff base-coumarin hybrids as potent α-glucosidase inhibitors. Chem Pap 74:4379–4388. https://doi.org/10.1007/s11696-020-01246-7
Sheta SM, Akl MA, Saad HE, El-Gharkawy ESRH (2020) A novel cerium(iii)-isatin Schiff base complex: spectrofluorometric and DFT studies and application as a kidney biomarker for ultrasensitive detection of human creatinine. RSC Adv 10:5853–5863. https://doi.org/10.1039/c9ra10133k
Shin E et al (2010) Inhibitory effects of coumarins from the stem barks of fraxinus rhynchophylla on adipocyte differentiation in 3T3-L1 cells. Biol Pharm Bull 33:1610–1614. https://doi.org/10.1248/bpb.33.1610
Shokohi-Pour Z, Chiniforoshan H, Sabzalian MR, Esmaeili S-A, Momtazi-borojeni AA (2018) Cobalt (II) complex with novel unsymmetrical tetradentate Schiff base (ON) ligand: in vitro cytotoxicity studies of complex, interaction with DNA/protein, molecular docking studies, and antibacterial activity. J Biomol Struct Dyn 36:532–549. https://doi.org/10.1080/07391102.2017.1287006
Singh H et al (2019) Rational approaches, design strategies, structure activity relationship and mechanistic insights for therapeutic coumarin hybrids. Bioorganic Med Chem 27:3477–3510. https://doi.org/10.1016/j.bmc.2019.06.033
Song KS, Liu L, Guo QX (2003) Remote substituent effects on N-X (X = H, F, Cl, CH3, Li) bond dissociation energies in Para-substituted anilines. J Org Chem 68:262–266. https://doi.org/10.1021/jo0204146
Sperry JB, Wright DL (2005) Furans, thiophenes and related heterocycles in drug discovery. Curr Opin Drug Discov Dev 8:723–740. https://doi.org/10.1002/chin.200615242
Spino C, Dodier M, Sotheeswaran S (1998) Anti-HIV coumarins from calophyllum seed oil. Bioorganic Med Chem Lett 8:3475–3478. https://doi.org/10.1016/S0960-894X(98)00628-3
Stephanos J (1996) Drug-protein interactions: Two-site binding of heterocyclic ligands to a monomeric hemoglobin. J Inorg Biochem 62:155–169. https://doi.org/10.1016/0162-0134(95)00144-1
Sudlow G, Birkett DJ, Wade DN (1976) Further characterization of specific drug binding sites on human serum albumin. Mol Pharmacol 12:1052–1061
Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K (1999) Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng Des Sel 12:439–446. https://doi.org/10.1093/protein/12.6.439
Teng CM, Lin CH, Ko FN, Wu TS, Huang TF (1994) The relaxant action of osthole isolated from Angelica pubescens in guinea-pig trachea. Naunyn Schmiedebergs Arch Pharmacol 349:202–208. https://doi.org/10.1007/BF00169838
Tramarin A et al (2019) New insights into the altered binding capacity of pharmaceutical-grade human serum albumin: site-specific binding studies by induced circular dichroism spectroscopy. J Pharm Biomed Anal 162:171–178. https://doi.org/10.1016/j.jpba.2018.09.022
Trott O, Olson AJ (2009) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. https://doi.org/10.1002/jcc.21334
Varshney A et al (2014) Analysis of binding interaction between antibacterial ciprofloxacin and human serum albumin by spectroscopic techniques. Cell Biochem Biophys 70:93–101. https://doi.org/10.1007/s12013-014-9863-1
Veber DF et al (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45:2615–2623. https://doi.org/10.1021/jm020017n
Verma A, Dewangan P, Kesharwani D, Kela SP (2013) Hypoglycemic and hypolipidemic activity of scopoletin (coumarin derivative) in streptozotocin induced diabetic rats. Int J Pharm Sci Rev Res 22:79–83
Vidya Rani C et al (2020) Bidentate Schiff base ligands appended metal(ii) complexes as probes of DNA and plasma protein: in silico molecular modelling studies. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-020-03270-5
Wang Q et al (2016) Binding interaction of atorvastatin with bovine serum albumin: spectroscopic methods and molecular docking. Spectrochim Acta Part A Mol Biomol Spectrosc 156:155–163. https://doi.org/10.1016/j.saa.2015.12.003
Wardell M et al (2002) The atomic structure of human methemalbumin at 1.9 Å. Biochem Biophys Res Commun 291:813–819. https://doi.org/10.1006/bbrc.2002.6540
Wenjing W et al (2019) Study on the interaction of ertugliflozin with human serum albumin in vitro by multispectroscopic methods, molecular docking, and molecular dynamics simulation . Spectrochim Acta Part A Mol Biomol Spectrosc 219:83–90. https://doi.org/10.1016/j.saa.2019.04.047
Whang WK et al (2005) Natural compounds, fraxin and chemicals structurally related to fraxin protect cells from oxidative stress. Exp Mol Med 37:436–446. https://doi.org/10.1038/emm.2005.54
Xavier S, Periandy S, Ramalingam S (2015) NBO, conformational, NLO, HOMO–LUMO, NMR and electronic spectral study on 1-phenyl-1-propanol by quantum computational methods. . Spectrochim Acta Part A Mol Biomol Spectrosc 137:306–320. https://doi.org/10.1016/j.saa.2014.08.039
Yadav P, Kumar Yadav J, Dixit AK, Agarwal A, Kumar AS (2019) Insight into the interaction of benzothiazole tethered triazole analogues with human serum albumin: spectroscopy and molecular docking approaches. Luminescence 34:812–822. https://doi.org/10.1002/bio.3676
Yi-Hui W et al (2019) Synthesis and antibacterial activity of novel chalcone derivatives bearing a coumarin moiety. Chem Pap 73:2493–2500. https://doi.org/10.1007/s11696-019-00802-0
Yousuf I, Bashir M, Arjmand F, Tabassum S (2019) Multispectroscopic insight, morphological analysis and molecular docking studies of Cu II-based chemotherapeutic drug entity with human serum albumin (HSA) and bovine serum albumin (BSA). J Biomol Struct Dyn 37:3290–3304. https://doi.org/10.1080/07391102.2018.1512899
Zaidi N, Ajmal MR, Rabbani G, Ahmad E, Khan RH (2013) A comprehensive insight into binding of hippuric acid to human serum albumin: a study to uncover its impaired elimination through hemodialysis Subramanyam, R, editor. PLoS ONE 8:e71422. https://doi.org/10.1371/journal.pone.0071422
Acknowledgements
The author is grateful to Taibah University for its support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parveen, S. Biophysical and theoretical investigation of benzo[c]coumarin functionalized Schiff base with human serum albumin. Chem. Pap. 75, 2339–2351 (2021). https://doi.org/10.1007/s11696-020-01496-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01496-5