Abstract
Purpose
Malarial parasites are susceptible to oxidative stress. The effects of α-tocopheryloxy acetic acid (α-TEA), a vitamin E analog, on infection by Plasmodium berghei ANKA and P. falciparum in mice and human red blood cells (RBCs), respectively, were examined in this study.
Methods
For in vivo studies in mice, RBCs infected with P. berghei ANKA were inoculated via intraperitoneal injection and α-TEA was administered to C57BL/6 J male mice after infection. The blood–brain barrier (BBB) permeability was examined by Evans blue staining in experimental cerebral malaria at 7 days after infection. The in vitro inhibitory effect of α-TEA on P. falciparum 3D7 (chloroquine-sensitive strain) and K1 (multidrug-resistant strain) was tested using a SYBR Green I-based assay.
Results
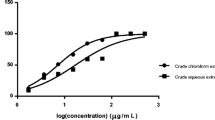
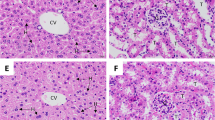
When 1.5% α-TEA was administered for 14 days after infection, 88% of P. berghei ANKA-infected mice survived during the experimental period. Nevertheless, all the control mice died within 12 days of infection. Furthermore, the Evans blue intensity in α-TEA-treated mice brains was less than that in untreated mice, indicating that α-TEA might inhibit the destruction of the BBB and progression of cerebral malaria. The in vitro experiment revealed that α-TEA inhibited the proliferation of both the 3D7 and K1 strains.
Conclusion
This study showed that α-TEA is effective against murine and human malaria in vivo and in vitro, respectively. Although α-TEA alone has a sufficient antimalarial effect, future research could focus on the structure–activity relationship to achieve better pharmacokinetics and decrease the cytotoxicity and/or the combined effect of α-TEA with existing drugs. In addition, the prophylactic antimalarial activity of premedication with α-TEA may also be an interesting perspective in the future.



Similar content being viewed by others
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Friedman MJ (1979) Oxidant damage mediates variant red cell resistance to malaria. Nature 280:245–247. https://doi.org/10.1038/280245a0
Clark IA, Hunt NH (1983) Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun 39:1–6. https://doi.org/10.1128/iai.39.1.1-6.1983
Gunjan S, Singh SK, Sharma T, Dwivedi H, Chauhan BS, Imran Siddiqi M, Tripathi R (2016) Mefloquine induces ROS mediated programmed cell death in malaria parasite: Plasmodium. Apoptosis 21:955–964. https://doi.org/10.1007/s10495-016-1265-y
Kline K, Yu W, Sanders BG (2004) Vitamin E and breast cancer. J Nutr 134(Supplement):3458S-3462S. https://doi.org/10.1093/jn/134.12.3458S
Neuzil J, Dong LF, Ramanathapuram L, Hahn T, Chladova M, Wang XF et al (2007) Vitamin E analogues as a novel group of mitocans: anti-cancer agents that act by targeting mitochondria. Mol Aspects Med 28:607–645. https://doi.org/10.1016/j.mam.2007.02.003
Wang XF, Witting PK, Salvatore BA, Neuzil J (2005) Vitamin E analogs trigger apoptosis in HER2/erbB2-overexpressing breast cancer cells by signaling via the mitochondrial pathway. Biochem Biophys Res Commun 326:282–289. https://doi.org/10.1016/j.bbrc.2004.11.028
Wang XF, Birringer M, Dong LF, Veprek P, Low P, Swettenham E et al (2007) A peptide conjugate of vitamin E succinate targets breast cancer cells with high erbB2 Expression. Cancer Res 67:3337–3344. https://doi.org/10.1158/0008-5472.CAN-06-2480
Qian M, Sanders BG, Kline K (1996) RRR-alpha-tocopheryl succinate induces apoptosis in avian retrovirus-transformed lymphoid cells. Nutr Cancer 25:9–26. https://doi.org/10.1080/01635589609514424
Neuzil J, Weber T, Schröder A, Lu M, Ostermann G, Gellert N et al (2001) Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. FASEB J 15:403–415. https://doi.org/10.1096/fj.00-0251com
Weber T, Lu M, Andera L, Lahm H, Gellert N, Fariss MW et al (2002) Vitamin E succinate is a potent novel antineoplastic agent with high selectivity and cooperativity with tumor necrosis factor-related apoptosis-inducing ligand (apo2 ligand) in vivo. Clin Cancer Res 8:863–869
Weber T, Dalen H, Andera L, Nègre-Salvayre A, Augé N, Sticha M et al (2003) Mitochondria play a central role in apoptosis induced by alpha-tocopheryl succinate, an agent with antineoplastic activity: comparison with receptor-mediated pro-apoptotic signaling. Biochemistry 42:4277–4291. https://doi.org/10.1021/bi020527j
Dong LF, Low P, Dyason JC, Wang XF, Prochazka L, Witting PK et al (2008) Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene 27:4324–4335. https://doi.org/10.1038/onc.2008.69
Dong LF, Freeman R, Liu J, Zobalova R, Marin-Hernandez A, Stantic M et al (2009) Suppression of tumor growth in vivo by the mitocan alpha-tocopheryl succinate requires respiratory complex II. Clin Cancer Res 15:1593–1600. https://doi.org/10.1158/1078-0432.CCR-08-2439
Bellezza I, Grottelli S, Gatticchi L, Mierla AL, Minelli A (2014) α-Tocopheryl succinate pre-treatment attenuates quinone toxicity in prostate cancer PC3 cells. Gene 539:1–7. https://doi.org/10.1016/j.gene.2014.02.009
Yan B, Stantic M, Zobalova R, Bezawork-Geleta A, Stapelberg M, Stursa J et al (2015) Mitochondrially targeted vitamin E succinate efficiently kills breast tumour-initiating cells in a complex II-dependent manner. BMC Cancer 15:401. https://doi.org/10.1186/s12885-015-1394-7
Neuzil J (2002) Alpha-tocopheryl succinate epitomizes a compound with a shift in biological activity due to pro-vitamin-to-vitamin conversion. Biochem Biophys Res Commun 293:1309–1313. https://doi.org/10.1016/S0006-291X(02)00358-3
Kume A, Kasai S, Furuya H, Suzuki H (2018) α-Tocopheryl succinate-suppressed development of cerebral malaria in mice. Parasitol Res 117:3177–3182. https://doi.org/10.1007/s00436-018-6016-2
Dong LF, Grant G, Massa H, Zobalova R, Akporiaye E, Neuzil J (2012) α-Tocopheryloxyacetic acid is superior to α-tocopheryl succinate in suppressing HER2-high breast carcinomas due to its higher stability. Int J Cancer 131:1052–1058. https://doi.org/10.1002/ijc.26489
Kawamura K, Kume A, Umemiya-Shirafuji R, Kasai S, Suzuki H (2021) Effect of α-tocopheryloxy acetic acid, a vitamin E derivative mitocan, on the experimental infection of mice with Plasmodium yoelii. Malar J 20:280. https://doi.org/10.1186/s12936-021-03817-9
Lawson KA, Anderson K, Menchaca M, Atkinson J, Sun L, Knight V et al (2003) Novel vitamin E analogue decreases syngeneic mouse mammary tumor burden and reduces lung metastasis. Mo Cancer Ther 2:437–444
Hahn T, Szabo L, Gold M, Ramanathapuram L, Hurley LH, Akporiaye ET (2006) Dietary administration of the proapoptotic vitamin E analogue α-tocopheryloxyacetic acid inhibits metastatic murine breast cancer. Cancer Res 66:9374–9378. https://doi.org/10.1158/0008-5472.CAN-06-2403
Jia L, Yu W, Wang P, Sanders BG, Kline K (2008) In vivo and in vitro studies of anticancer actions of alpha-TEA for human prostate cancer cells. Prostate 68:849–860. https://doi.org/10.1002/pros.20750
Li Y, Hahn T, Garrison K, Cui ZH, Thorburn A, Thorburn J et al (2012) The vitamin E analogue α-TEA stimulates tumor autophagy and enhances antigen cross-presentation. Cancer Res 72:3535–3545. https://doi.org/10.1158/0008-5472.CAN-11-3103
Yao J, Gao P, Xu Y, Li Z (2016) α-TEA inhibits the growth and motility of human colon cancer cells via targeting RhoA/ROCK signaling. Mol Med Rep 14:2534–2540. https://doi.org/10.3892/mmr.2016.5525
Yu W, Tiwary R, Li J, Park SK, Jia L, Xiong A et al (2010) α-TEA induces apoptosis of human breast cancer cells via activation of TRAIL/DR5 death receptor pathway. Mol Carcinog 49:964–973. https://doi.org/10.1002/mc.20681
Rodríguez-Enríquez S, Hernández-Esquivel L, Marín-Hernández A, Dong LF, Akporiaye ET, Neuzil J et al (2012) Molecular mechanism for the selective impairment of cancer mitochondrial function by a mitochondrially targeted vitamin E analogue. Biochim Biophys Acta 1817:1597–1607. https://doi.org/10.1016/j.bbabio.2012.05.005
Leesombun A, Iijima M, Pagmadulam B, Orkhon B, Doi H, Issiki K et al (2021) Metacytofilin has potent anti-malarial activity. Parasitol Int 81:102267. https://doi.org/10.1016/j.parint.2020.102267
Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M (2004) Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806. https://doi.org/10.1128/AAC.48.5.1803-1806.2004
Evans BC, Nelson CE, Yu SS, Beavers KR, Kim AJ, Li H et al (2013) Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J Vis Exp 73:e50166. https://doi.org/10.3791/50166
Hahn T, Akporiaye ET (2013) α-TEA as a stimulator of tumor autophagy and enhancer of antigen cross-presentation. Autophagy 9:429–431. https://doi.org/10.4161/auto.22969
Herbas MS, Ueta YY, Ichikawa C, Chiba M, Ishibashi K, Shichiri M, Fukumoto S, Yokoyama N, Takeya M, Xuan X, Arai H, Suzuki H (2010) Alpha-tocopherol transfer protein disruption confers resistance to malarial infection in mice. Malar J 9:101. https://doi.org/10.1186/1475-2875-9-101.PMID:20403155;PMCID:PMC2862040
Herbas MS, Shichiri M, Ishida N, Kume A, Hagihara Y, Yoshida Y, Suzuki H (2015) Probucol-induced α-tocopherol deficiency protects mice against malaria infection. PLoS ONE 10:e0136014. https://doi.org/10.1371/journal.pone.0136014.PMID:26296197;PMCID:PMC4546625
Kume A, Anh DT, Shichiri M, Ishida N, Suzuki H (2016) Probucol dramatically enhances dihydroartemisinin effect in murine malaria. Malar J 15:472. https://doi.org/10.1186/s12936-016-1532-y
de Sena Pereira VS, Silva de Oliveira CB, Fumagalli F, da Silva EF, da Silva NB, de Andrade-Neto VF (2016) Cytotoxicity, hemolysis and in vivo acute toxicity of 2-hydroxy-3-anilino-1,4-naphthoquinone derivatives. Toxicol Reports 2016(3):756–762. https://doi.org/10.1016/j.toxrep.2016.09.007
Hahn T, Akporiaye ET (2012) Repeat dose study of the novel proapoptotic chemotherapeutic agent alpha-tocopheryloxy acetic acid in mice. Anti Cancer Drugs 23:455–464. https://doi.org/10.1097/CAD.0b013e32834f6271
Kawamura K, Suzuki H (2022) Unpublished work.
Dong LF, Swettenham E, Eliasson J, Wang XF, Gold M, Medunic Y et al (2007) Vitamin E analogues inhibit angiogenesis by selective induction of apoptosis in proliferating endothelial cells: the role of oxidative stress. Cancer Res 67:11906–11913. https://doi.org/10.1158/0008-5472.CAN-07-3034
Acknowledgements
The authors would like to thank Editage (www.editage.com) for English language editing.
Funding
A part of this work was supported by grant from the Naito Foundation.
Author information
Authors and Affiliations
Contributions
The research was designed by HS, AK, YN, and SK. Laboratory experiments were performed by NA and AK The manuscript was written by HS, TT, YN, and RU-S. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics Approval
The animals used in this study were treated and cared for based on the Guiding Principles for the Care and Use of Research Animals established by Obihiro University of Agriculture and Veterinary Medicine. All animal experimental protocols were approved by the Institutional Animal Ethics Committee, Obihiro University of Agriculture and Veterinary Medicine.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ariefta, N.R., Kume, A., Nishikawa, Y. et al. Effect of α-Tocopheryloxy Acetic Acid on the Infection of Mice with Plasmodium berghei ANKA In Vivo and Humans with P. falciparum In Vitro. Acta Parasit. 67, 1514–1520 (2022). https://doi.org/10.1007/s11686-022-00604-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00604-7