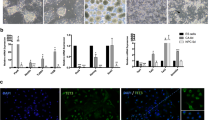
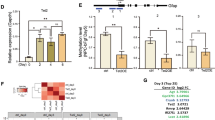
Abstract
Brain development requires a delicate balance between self-renewal and differentiation in neural stem cells (NSC), which rely on the precise regulation of gene expression. Ten-eleven translocation 2 (TET2) modulates gene expression by the hydroxymethylation of 5-methylcytosine in DNA as an important epigenetic factor and participates in the neuronal differentiation. Yet, the regulation of TET2 in the process of neuronal differentiation remains unknown. Here, the protein level of TET2 was reduced by the ubiquitin-proteasome pathway during NSC differentiation, in contrast to mRNA level. We identified that TET2 physically interacts with the core subunits of the glucose-induced degradation-deficient (GID) ubiquitin ligase complex, an evolutionarily conserved ubiquitin ligase complex and is ubiquitinated by itself. The protein levels of GID complex subunits increased reciprocally with TET2 level upon NSC differentiation. The silencing of the core subunits of the GID complex, including WDR26 and ARMC8, attenuated the ubiquitination and degradation of TET2, increased the global 5-hydroxymethylcytosine levels, and promoted the differentiation of the NSC. TET2 level increased in the brain of the Wdr26+/− mice. Our results illustrated that the GID complex negatively regulates TET2 protein stability, further modulates NSC differentiation, and represents a novel regulatory mechanism involved in brain development.
Similar content being viewed by others
References
Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011; 333(6047): 1300–1303
He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Sun Y, Li X, Dai Q, Song CX, Zhang K, He C, Xu GL. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011; 333(6047): 1303–1307
Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet 2017; 18(9): 517–534
Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky G, Lahesmaa R, Orkin SH, Rodig SJ, Daley GQ, Rao A. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell 2011; 8(2): 200–213
Dawlaty MM, Ganz K, Powell BE, Hu YC, Markoulaki S, Cheng AW, Gao Q, Kim J, Choi SW, Page DC, Jaenisch R. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell 2011; 9(2): 166–175
Li T, Yang D, Li J, Tang Y, Yang J, Le W. Critical role of Tet3 in neural progenitor cell maintenance and terminal differentiation. Mol Neurobiol 2015; 51(1): 142–154
Li X, Yao B, Chen L, Kang Y, Li Y, Cheng Y, Li L, Lin L, Wang Z, Wang M, Pan F, Dai Q, Zhang W, Wu H, Shu Q, Qin Z, He C, Xu M, Jin P. Ten-eleven translocation 2 interacts with forkhead box O3 and regulates adult neurogenesis. Nat Commun 2017; 8: 15903
Zhang Q, Hu Q, Wang J, Miao Z, Li Z, Zhao Y, Wan B, Allen EG, Sun M, Jin P, Xu X. Stress modulates Ahi1-dependent nuclear localization of ten-eleven translocation protein 2. Hum Mol Genet 2021; 30(22): 2149–2160
Li L, Miao M, Chen J, Liu Z, Li W, Qiu Y, Xu S, Wang Q. Role of Ten eleven translocation-2 (Tet2) in modulating neuronal morphology and cognition in a mouse model of Alzheimer’s disease. J Neurochem 2021; 157(4): 993–1012
Mi Y, Gao X, Dai J, Ma Y, Xu L, Jin W. A Novel function of TET2 in CNS: sustaining neuronal survival. Int J Mol Sci 2015; 16(9): 21846–21857
Wang Y, Zhang Y. Regulation of TET protein stability by calpains. Cell Rep 2014; 6(2): 278–284
Cheng J, Guo S, Chen S, Mastriano SJ, Liu C, D’Alessio AC, Hysolli E, Guo Y, Yao H, Megyola CM, Li D, Liu J, Pan W, Roden CA, Zhou XL, Heydari K, Chen J, Park IH, Ding Y, Zhang Y, Lu J. An extensive network of TET2-targeting microRNAs regulates malignant hematopoiesis. Cell Rep 2013; 5(2): 471–481
Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, Rabidou K, Fang R, Tan L, Xu S, Liu H, Argueta C, Zhang L, Mao F, Yan G, Chen J, Dong Z, Lv R, Xu Y, Wang M, Ye Y, Zhang S, Duquette D, Geng S, Yin C, Lian CG, Murphy GF, Adler GK, Garg R, Lynch L, Yang P, Li Y, Lan F, Fan J, Shi Y, Shi YG. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 2018; 559(7715): 637–641
Ko M, An J, Bandukwala HS, Chavez L, Aijö T, Pastor WA, Segal MF, Li H, Koh KP, Lähdesmäki H, Hogan PG, Aravind L, Rao A. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature 2013; 497(7447): 122–126
Lv L, Wang Q, Xu Y, Tsao LC, Nakagawa T, Guo H, Su L, Xiong Y. Vpr targets TET2 for degradation by CRL4VprBP E3 ligase to sustain IL-6 expression and enhance HIV-1 replication. Mol Cell 2018; 70(5): 961–970.e5
Santt O, Pfirrmann T, Braun B, Juretschke J, Kimmig P, Scheel H, Hofmann K, Thumm M, Wolf DH. The yeast GID complex, a novel ubiquitin ligase (E3) involved in the regulation of carbohydrate metabolism. Mol Biol Cell 2008; 19(8): 3323–3333
Lampert F, Stafa D, Goga A, Soste MV, Gilberto S, Olieric N, Picotti P, Stoffel M, Peter M. The multi-subunit GID/CTLH E3 ubiquitin ligase promotes cell proliferation and targets the transcription factor Hbp1 for degradation. eLife 2018; 7: e35528
Skraban CM, Wells CF, Markose P, Cho MT, Nesbitt AI, Au PYB, Begtrup A, Bernat JA, Bird LM, Cao K, de Brouwer APM, Denenberg EH, Douglas G, Gibson KM, Grand K, Goldenberg A, Innes AM, Juusola J, Kempers M, Kinning E, Markie DM, Owens MM, Payne K, Person R, Pfundt R, Stocco A, Turner CLS, Verbeek NE, Walsh LE, Warner TC, Wheeler PG, Wieczorek D, Wilkens AB, Zonneveld-Huijssoon E; Deciphering Developmental Disorders Study; Kleefstra T, Robertson SP, Santani A, van Gassen KLI, Deardorff MA. WDR26 haploinsufficiency causes a recognizable syndrome of intellectual disability, seizures, abnormal gait, and distinctive facial features. Am J Hum Genet 2017; 101(1): 139–148
Córdova-Palomera A, Fatjó-Vilas M, Gastó C, Navarro V, Krebs MO, Fañanás L. Genome-wide methylation study on depression: differential methylation and variable methylation in monozygotic twins. Transl Psychiatry 2015; 5(4): e557
Tangsuwansri C, Saeliw T, Thongkorn S, Chonchaiya W, Suphapeetiporn K, Mutirangura A, Tencomnao T, Hu VW, Sarachana T. Investigation of epigenetic regulatory networks associated with autism spectrum disorder (ASD) by integrated global LINE-1 methylation and gene expression profiling analyses. PLoS One 2018; 13(7): e0201071
Dong C, Zhang H, Li L, Tempel W, Loppnau P, Min J. Molecular basis of GID4-mediated recognition of degrons for the Pro/N-end rule pathway. Nat Chem Biol 2018; 14(5): 466–473
Zhang YW, Wang Z, Xie W, Cai Y, Xia L, Easwaran H, Luo J, Yen RC, Li Y, Baylin SB. Acetylation enhances TET2 function in protecting against abnormal DNA methylation during oxidative stress. Mol Cell 2017; 65(2): 323–335
Guallar D, Bi X, Pardavila JA, Huang X, Saenz C, Shi X, Zhou H, Faiola F, Ding J, Haruehanroengra P, Yang F, Li D, Sanchez-Priego C, Saunders A, Pan F, Valdes VJ, Kelley K, Blanco MG, Chen L, Wang H, Sheng J, Xu M, Fidalgo M, Shen X, Wang J. RNA-dependent chromatin targeting of TET2 for endogenous retrovirus control in pluripotent stem cells. Nat Genet 2018; 50(3): 443–451
Sun Z, Smrcka AV, Chen S. WDR26 functions as a scaffolding protein to promote Gβγ-mediated phospholipase C β2 (PLCβ2) activation in leukocytes. J Biol Chem 2013; 288(23): 16715–16725
Chen SJ, Wu X, Wadas B, Oh JH, Varshavsky A. An N-end rule pathway that recognizes proline and destroys gluconeogenic enzymes. Science 2017; 355(6323): eaal3655
Hochgerner H, Zeisel A, Lönnerberg P, Linnarsson S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat Neurosci 2018; 21(2): 290–299
Muhr J, Hagey DW. The cell cycle and differentiation as integrated processes: cyclins and CDKs reciprocally regulate Sox and Notch to balance stem cell maintenance. BioEssays 2021; 43(7): e2000285
Borlongan CV. Regenerative medicine during the pandemic period. Brain Circ 2021; 7(1): 1–2
Farkas LM, Huttner WB. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr Opin Cell Biol 2008; 20(6): 707–715
Guo Z, Chen M, Chao Y, Cai C, Liu L, Zhao L, Li L, Bai QR, Xu Y, Niu W, Shi L, Bi Y, Ren D, Yuan F, Shi S, Zeng Q, Han K, Shi Y, Bian S, He G. RGCC balances self-renewal and neuronal differentiation of neural stem cells in the developing mammalian neocortex. EMBO Rep 2021; 22(9): e51781
Gilmore EC, Walsh CA. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol 2013; 2(4): 461–478
Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci USA 2006; 103(1): 111–116
Wilpert NM, Marguet F, Maillard C, Guimiot F, Martinovic J, Drunat S, Attié-Bitach T, Razavi F, Tessier A, Capri Y, Laquerrière A, Bahi-Buisson N. Human neuropathology confirms projection neuron and interneuron defects and delayed oligodendrocyte production and maturation in FOXG1 syndrome. Eur J Med Genet 2021; 64(9): 104282
Gruber R, Zhou Z, Sukchev M, Joerss T, Frappart PO, Wang ZQ. MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat Cell Biol 2011; 13(11): 1325–1334
Maraldi T, Angeloni C, Prata C, Hrelia S. NADPH oxidases: redox regulators of stem cell fate and function. antioxidants 2021; 10(6): 973
Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, Ye P, Rosa L, Lee YK, Wu HP, Liu W, Xu ZM, Yang L, Ding YQ, Tang F, Meissner A, Ding C, Shi Y, Xu GL. Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell 2013; 13(2): 237–245
Izumi K. Disorders of transcriptional regulation: an emerging category of multiple malformation syndromes. Mol Syndromol 2016; 7(5): 262–273
Bestman JE, Huang LC, Lee-Osbourne J, Cheung P, Cline HT. An in vivo screen to identify candidate neurogenic genes in the developing Xenopus visual system. Dev Biol 2015; 408(2): 269–291
Nassan M, Li Q, Croarkin PE, Chen W, Colby CL, Veldic M, McElroy SL, Jenkins GD, Ryu E, Cunningham JM, Leboyer M, Frye MA, Biernacka JM. A genome wide association study suggests the association of muskelin with early onset bipolar disorder: implications for a GABAergic epileptogenic neurogenesis model. J Affect Disord 2017; 208: 120–129
Huffman N, Palmieri D, Coppola V. The CTLH complex in cancer cell plasticity. J Oncol 2019; 2019: 4216750
Acknowledgements
We thank Professor Caiyong Chen from College of Life Sciences, Zhejiang University for providing the FLAG-ARMC8 and FLAG-WDR26 plasmids. This study was supported by the National Science Foundation of China (Nos. 82071511 and 81120108011), National Key R&D Program of China (No. 2017YFE0103700), Shandong Provincial Natural Science Foundation (No. ZR2019ZD32), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX21_2974).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest Meiling Xia, Rui Yan, Wenjuan Wang, Meng Zhang, Zhigang Miao, Bo Wan, and Xingshun Xu declare that they have no competing interests.
All animal use protocols were approved by the institutional animal care and use committee of Soochow University. All institutional and national guidelines for the care and use of laboratory animals were followed.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xia, M., Yan, R., Wang, W. et al. GID complex regulates the differentiation of neural stem cells by destabilizing TET2. Front. Med. 17, 1204–1218 (2023). https://doi.org/10.1007/s11684-023-1007-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-023-1007-9