Abstract
Background
The amygdala plays a crucial role in the central pathogenesis mechanism of primary dysmenorrhea (PDM). However, the detailed pain modulation principles of the amygdala in PDM remain unclear. Here, we applied the Granger causality analysis (GCA) to investigate the directional effective connectivity (EC) alterations in the amygdala network of PDM patients.
Methods
Thirty-seven patients with PDM and 38 healthy controls were enrolled in this study and underwent resting-state functional magnetic resonance imaging scans during the pain-free stage. GCA was employed to explore the amygdala-based EC network alteration in PDM. A multivariate pattern analysis (MVPA)-based machine learning approach was used to explore whether the altered amygdala EC could serve as an fMRI-based marker for classifying PDM and HC participants.
Results
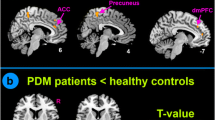
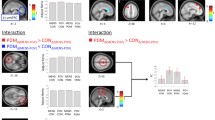
Compared to the healthy control group, patients with PDM showed significantly decreased EC from the amygdala to the right superior frontal gyrus (SFG), right superior parietal lobe/middle occipital gyrus, and left middle cingulate cortex, whereas increased EC was found from the amygdala to the bilateral medial orbitofrontal cortex. In addition, increased EC was found from the bilateral SFG to the amygdala, and decreased EC was found from the medial orbitofrontal cortex, caudate nucleus to the amygdala. The increased EC from the right SFG to the amygdala was associated with a plasma prostaglandin E2 level in PDM. The MVPA based on an altered amygdala EC pattern yielded a total accuracy of 86.84% for classifying the patients with PDM and HC.
Conclusion
Our study is the first to combine MVPA and EC to explore brain function alteration in PDM. The results could advance understanding of the neural theory of PDM in specifying the pain-free period.




Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings cannot be shared as the data also form part of an ongoing study.
References
Allen, H. N., Bobnar, H. J., & Kolber, B. J. (2021). Left and right hemispheric lateralization of the amygdala in pain. Progress in Neurobiology, 196, 101891. https://doi.org/10.1016/j.pneurobio.2020.101891
Baajour, S. J., Chowdury, A., Thomas, P., Rajan, U., Khatib, D., Zajac-Benitez, C., … & Diwadkar, V. A. (2020). Disordered directional brain network interactions during learning dynamics in schizophrenia revealed by multivariate autoregressive models. Human Brain Mapping, 41(13), 3594–3607. https://doi.org/10.1002/hbm.25032
Boissoneault, J., Sevel, L., Letzen, J., Robinson, M., & Staud, R. (2017). Biomarkers for musculoskeletal pain conditions: Use of brain imaging and machine learning. Current Rheumatology Reports, 19(1), 5. https://doi.org/10.1007/s11926-017-0629-9
Burnett, M., & Lemyre, M. (2017). No. 345-primary dysmenorrhea consensus guideline. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC, 39(7), 585–595. https://doi.org/10.1016/j.jogc.2016.12.023
Burnett, M. A., Antao, V., Black, A., Feldman, K., Grenville, A., Lea, R., … Robert, M. (2005). Prevalence of Primary Dysmenorrhea in Canada. Journal of Obstetrics and Gynaecology Canada, 27(8), 765–770. https://doi.org/10.1016/S1701-2163(16)30728-9
Chan, W. Y. (1983). Prostaglandins and nonsteroidal antiinflammatory drugs in dysmenorrhea. Annual Review of Pharmacology and Toxicology, 23, 131–149. Retrieved from https://pubmed.ncbi.nlm.nih.gov/6347048
Chang, C.-C., & Lin, C.-J. (2011). LIBSVM: A library for support vector machines. ACM Transactions on Intelligent Systems and Technology (TIST), 2(3), 1–27.
Coco, A. S. (1999). Primary dysmenorrhea. American Family Physician, 60(2), 489–496.
Cottam, W. J., Iwabuchi, S. J., Drabek, M. M., Reckziegel, D., & Auer, D. P. (2018). Altered connectivity of the right anterior insula drives the pain connectome changes in chronic knee osteoarthritis. Pain, 159(5), 929–938. https://doi.org/10.1097/j.pain.0000000000001209
Diekhof, E. K., Kaps, L., Falkai, P., & Gruber, O. (2012). The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude – An activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia, 50(7), 1252–1266. https://doi.org/10.1016/j.neuropsychologia.2012.02.007
du Boisgueheneuc, F., Levy, R., Volle, E., Seassau, M., Duffau, H., Kinkingnehun, S., … Dubois, B. (2006). Functions of the left superior frontal gyrus in humans: a lesion study. Brain, 129(Pt 12), 3315–3328. https://doi.org/10.1093/brain/awl244
Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity, 1(1), 13–36. https://doi.org/10.1089/brain.2011.0008
Haxby, J. V. (2012). Multivariate pattern analysis of fMRI: The early beginnings. NeuroImage, 62(2), 852–855. https://doi.org/10.1016/j.neuroimage.2012.03.016
Holland, P. C., & Gallagher, M. (2004). Amygdala-frontal interactions and reward expectancy. Current Opinion in Neurobiology, 14(2), 148–155. https://doi.org/10.1016/j.conb.2004.03.007
Hou, Z., Gong, L., Zhi, M., Yin, Y., Zhang, Y., Xie, C., & Yuan, Y. (2018). Distinctive pretreatment features of bilateral nucleus accumbens networks predict early response to antidepressants in major depressive disorder. Brain Imaging and Behavior, 12(4), 1042–1052. https://doi.org/10.1007/s11682-017-9773-0
Hu, L., & Iannetti, G. D. (2016). Painful issues in pain prediction. Trends in Neurosciences, 39(4), 212–220. https://doi.org/10.1016/j.tins.2016.01.004
Huang, X., Zhang, D., Wang, P., Mao, C., Miao, Z., Liu, C., … Wu, X. (2021). Altered amygdala effective connectivity in migraine without aura: evidence from resting‐state fMRI with Granger causality analysis. The Journal of Headache and Pain, 22(1), 1-8.
Iacovides, S., Avidon, I., & Baker, F. C. (2015). What we know about primary dysmenorrhea today: A critical review. Human Reproduction Update, 21(6), 762–778. https://doi.org/10.1093/humupd/dmv039
Jensen, D. V., Andersen, K. B., & Wagner, G. (1987). Prostaglandins in the menstrual cycle of women. A review. Danish Medical Bulletin, 34(3), 178–182.
Ji, G., Sun, H., Fu, Y., Li, Z., Pais-Vieira, M., Galhardo, V., & Neugebauer, V. (2010). Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. Journal of Neuroscience, 30(15), 5451–5464. https://doi.org/10.1523/jneurosci.0225-10.2010
Kawabata, A. (2011). Prostaglandin E2 and pain–an update. Biological &/and Pharmaceutical Bulletin, 34(8), 1170–1173. https://doi.org/10.1248/bpb.34.1170
Kim, E., Kim, D. S., Ahmad, F., & Park, H. (2013). Pattern-based Granger causality mapping in FMRI. Brain Connect, 3(6), 569–577. https://doi.org/10.1089/brain.2013.0148
Larroy, C. (2002). Comparing visual-analog and numeric scales for assessing menstrual pain. Behavioral Medicine, 27(4), 179–181.
Liu, P., Yang, J., Wang, G., Liu, Y., Liu, X., Jin, L., … Calhoun, V. D. (2016). Altered regional cortical thickness and subcortical volume in women with primary dysmenorrhoea. European Journal of Pain, 20(4), 512–520.
Liu, Q., Zeng, X.-C., Jiang, X.-M., Zhou, Z.-H., & Hu, X.-F. (2019). Altered brain functional hubs and connectivity underlie persistent somatoform pain disorder. Frontiers in Neuroscience, 13, 415. https://doi.org/10.3389/fnins.2019.00415
Low, I., Wei, S.-Y., Lee, P.-S., Li, W.-C., Lee, L.-C., Hsieh, J.-C., & Chen, L.-F. (2018). Neuroimaging studies of primary dysmenorrhea. Advances in Experimental Medicine and Biology, 1099, 179–199. https://doi.org/10.1007/978-981-13-1756-9_16
Marjoribanks, J., Ayeleke, R. O., Farquhar, C., & Proctor, M. (2015). Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev, 2015(7), Cd001751. https://doi.org/10.1002/14651858.CD001751.pub3
Neugebauer, V. (2015). Amygdala pain mechanisms. Handbook of Experimental Pharmacology, 227, 261–284. https://doi.org/10.1007/978-3-662-46450-2_13
Neugebauer, V., Li, W., Bird, G. C., & Han, J. S. (2004). The amygdala and persistent pain. The Neuroscientist, 10(3), 221–234. https://doi.org/10.1177/1073858403261077
Ojala, M., & Garriga, G. C. (2010). Permutation tests for studying classifier performance. Journal of Machine Learning Research, 11(6).
Quan, S., Yang, J., Dun, W., Wang, K., Liu, H., & Liu, J. (2020). Prediction of pain intensity with uterine morphological features and brain microstructural and functional properties in women with primary dysmenorrhea. Brain Imaging Behav, 1–9.
RC, O. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
Rolls, E. T., Cheng, W., & Feng, J. (2020). The orbitofrontal cortex: reward, emotion and depression. Brain Commun, 2(2), fcaa196. https://doi.org/10.1093/braincomms/fcaa196
Schultz, W. (2016). Reward functions of the basal ganglia. Journal of Neural Transmission (vienna), 123(7), 679–693. https://doi.org/10.1007/s00702-016-1510-0
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 27(9), 2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Seth, A. K., Barrett, A. B., & Barnett, L. (2015). Granger causality analysis in neuroscience and neuroimaging. Journal of Neuroscience, 35(8), 3293–3297.
Shen, W., Tu, Y., Gollub, R. L., Ortiz, A., Napadow, V., Yu, S., … Jung, M. (2019a). Visual network alterations in brain functional connectivity in chronic low back pain: A resting state functional connectivity and machine learning study. NeuroImage: Clinical, 22, 101775.
Shen, Z., Yu, S., Wang, M., She, T., Yang, Y., Wang, Y., … Liang, F. (2019b). Abnormal amygdala resting-state functional connectivity in primary dysmenorrhea. Neuroreport, 30(5), 363–368.
Simons, L. E., Moulton, E. A., Linnman, C., Carpino, E., Becerra, L., & Borsook, D. (2014). The human amygdala and pain: Evidence from neuroimaging. Human Brain Mapping, 35(2), 527–538. https://doi.org/10.1002/hbm.22199
Szczepanski, S. M., Pinsk, M. A., Douglas, M. M., Kastner, S., & Saalmann, Y. B. (2013). Functional and structural architecture of the human dorsal frontoparietal attention network. Proceedings of the National Academy of Sciences, 110(39), 15806–15811. https://doi.org/10.1073/pnas.1313903110
Tang, W., Bressler, S. L., Sylvester, C. M., Shulman, G. L., & Corbetta, M. (2012). Measuring Granger causality between cortical regions from voxelwise fMRI BOLD signals with LASSO. PLoS Computational Biology, 8(5), e1002513. https://doi.org/10.1371/journal.pcbi.1002513
Thompson, J. M., & Neugebauer, V. (2017). Amygdala plasticity and pain. Pain Research & Management.
Tu, Y., Ortiz, A., Gollub, R. L., Cao, J., Gerber, J., Lang, C., … Kong, J. (2019). Multivariate resting-state functional connectivity predicts responses to real and sham acupuncture treatment in chronic low back pain. Neuroimaging Clinics, 23, 101885. https://doi.org/10.1016/j.nicl.2019.101885
Tu, Y., Zeng, F., Lan, L., Li, Z., Maleki, N., Liu, B., … Lang, C. (2020). An fMRI-based neural marker for migraine without aura. Neurology, 94(7), e741–e751.
Varfolomeev, S. D., Semenova, N. A., Bykov, V. I., & Tsybenova, S. B. (2019). Kinetics of chemical processes in the human brain: Modeling of the BOLD fMRI signal. Doklady Physical Chemistry, 488(1), 125–128. https://doi.org/10.1134/S0012501619090057
Wager, T. D., Atlas, L. Y., Lindquist, M. A., Roy, M., Woo, C. W., & Kross, E. (2013). An fMRI-based neurologic signature of physical pain. New England Journal of Medicine, 368(15), 1388–1397. https://doi.org/10.1056/NEJMoa1204471
Wei, H.-L., Chen, J., Chen, Y.-C., Yu, Y.-S., Guo, X., Zhou, G.-P., … Zhang, H. (2020). Impaired effective functional connectivity of the sensorimotor network in interictal episodic migraineurs without aura. The Journal of Headache and Pain, 21(1), 111. https://doi.org/10.1186/s10194-020-01176-5
Yang, L., Dun, W., Li, K., Yang, J., Wang, K., Liu, H., … Zhang, M. (2019). Altered amygdalar volume and functional connectivity in primary dysmenorrhoea during the menstrual cycle. European Journal of Pain (London, England), 23(5). https://doi.org/10.1002/ejp.1368
Yu, S., Li, W., Shen, W., Edwards, R. R., Gollub, R. L., Wilson, G., … Gerber, J. (2020a). Impaired mesocorticolimbic connectivity underlies increased pain sensitivity in chronic low back pain. Neuroimage, 218, 116969.
Yu, S., Ortiz, A., Gollub, R. L., Wilson, G., Gerber, J., Park, J., … Wasan, A. D. (2020b). Acupuncture treatment modulates the connectivity of key regions of the descending pain modulation and reward systems in patients with chronic low back pain. Journal of Clinical Medicine, 9(6), 1719.
Yu, S., Xie, M., Liu, S., Guo, X., Tian, J., Wei, W., … Yang, J. (2020c). Resting-State Functional Connectivity Patterns Predict Acupuncture Treatment Response in Primary Dysmenorrhea. Frontiers in Neuroscience, 14, 559191. https://doi.org/10.3389/fnins.2020.559191
Zang, Z.-X., Yan, C.-G., Dong, Z.-Y., Huang, J., & Zang, Y.-F. (2012). Granger causality analysis implementation on MATLAB: A graphic user interface toolkit for fMRI data processing. Journal of Neuroscience Methods, 203(2), 418–426.
Zhang, Q., Yu, S., Wang, Y., Wang, M., Yang, Y., Wei, W., … Yang, J. (2019). Abnormal reward system network in primary dysmenorrhea. Molecular Pain, 15, 1744806919862096. https://doi.org/10.1177/1744806919862096
Zung, W. W. (1971). A rating instrument for anxiety disorders. Psychosomatics, 12(6), 371–379. https://doi.org/10.1016/S0033-3182(71)71479-0
Zung, W. W., Richards, C. B., & Short, M. J. (1965). Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Archives of General Psychiatry, 13(6), 508–515. Retrieved from https://pubmed.ncbi.nlm.nih.gov/4378854
Funding
This work was supported by the China National Postdoctoral Program for Innovative Talent (No. BX20190046), the National Natural Science Foundation of China (No. 81973966), the Science and Technology Support Program of Nanchong (19SXHZ0100), and the Doctoral Scientific Research Foundation of North Sichuan Medical College (CBY19-QD10).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
The current study was approved by the Institutional Review Board of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (CDUTCM), Chengdu, China. Written informed consent was obtained from each participant before enrollment.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1
(DOCX 13.4 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, S., Liu, L., Chen, L. et al. Classification of primary dysmenorrhea by brain effective connectivity of the amygdala: a machine learning study. Brain Imaging and Behavior 16, 2517–2525 (2022). https://doi.org/10.1007/s11682-022-00707-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-022-00707-9