Abstract
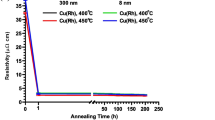
Ruthenium (Ru)—a high-melting-point precious metal—has attracted attention for use as ultrafine interconnections in large-scale integrations. This is because the resistivity of Ru interconnects is not expected to increase with a reduction in the interconnect width owing to their short mean free path for electrons. In this study, we investigated electroless plating of Ru using hydrazine hydrate as a reducing agent to obtain low-resistivity Ru films. We obtained polycrystalline Ru films on a thin (10-nm) catalytic chemical-vapor-deposited Ru underlayer. The electroless Ru films exhibited significant grain growth upon annealing at 600°C in a forming gas (N2:H2 = 9:1). The Ru (101) and (100) crystalline orientations were strengthened by annealing, and the resistivity decreased from 160 µΩ cm to 22 µΩ cm concomitantly. Thermal desorption spectroscopy showed that the electroless Ru films contained impurities, such as CO, CO2, NH3, O, C, and H2. Desorption of C, CO, CO2, and NH3 showed peak maxima at approximately 450–500 K. These impurity molecules likely came from the inclusion of the complexing agents (tartaric acid: C4(OH)4O2, and ammonium chloride: NH4Cl) and reducing agent (hydrazine: N2H2) into the Ru film. Desorption of these molecules during annealing may improve the grain growth of polycrystalline Ru and reduce its resistivity.









Similar content being viewed by others
References
C.-C. Yang, F.R. McFeely, P.-C. Wang, K. Chanda, and D.C. Edelstein, Selective chemical vapor deposition-grown Ru for Cu interconnect capping applications. Electrochem. Solid State Lett. 13–5, D33 (2010).
P.R. Gadkari, A.P. Warren, R.M. Todi, R.V. Petrova, and K.R. Coffey, Comparison of the agglomeration behavior of thin metallic films on SiO2. J. Vacuum. Sci. Technol. A23, 1152 (2005).
W.T. Lim and C.H. Lee, Highly oriented ZnO thin films deposited on Ru/Si substrates. Thin Solid Films 353, 12 (1999).
J.Z. Shi, S.N. Piramanayagam, C.S. Mah, H.B. Zhao, J.M. Zhao, Y.S. Kay, and C.K. Pock, Influence of dual-Ru intermediate layers on magnetic properties and recording performance of CoCrPt-SiO2 perpendicular recording media. Appl. Phys. Lett. 87(22), 222503 (2005).
Y. Huai, J. Zhang, G.W. Anderson, P. Rana, S. Funada, C.-Y. Hung, M. Zhao, and S. Tran, Spin-valve heads with synthetic antiferromagnet CoFe/Ru/CoFe/IrMn. J. Appl. Phys. 85, 5528 (1999).
J.-S. Jang, D.-J. Kim, S.-J. Park, and T.-Y. Seong, Electrical characteristics of thermally stable Ru and Ru/Au ohmic contacts to surface-treated p-type GaN. J. Electron. Mater. 35, 94 (2006).
J.H. Lin, J.H. Lee, C.-S. Hsu, and J.S. Fang, Fifteen-Nanometer Ru diffusion barrier on NiSi/Si for a sub 45 nm Cu contact plug. J. Electron. Mater. 38(11), 2251 (2009).
O. Chyan, T.N. Arunagiri, and T. Ponnuswamy, Electrodeposition of copper thin film on ruthenium: a potential diffusion barrier for Cu interconnects. J. Electrochem. Soc. 150, C347 (2003).
B.H. Choi, Y.H. Lim, J.H. Lee, B.K. Kim, H.-N. Lee, and H.K. Lee, Preparation of Ru thin film layer on Si and TaN/Si as diffusion barrier by plasma enhanced atomic layer deposition. Microelectron. Eng. 87, 1391 (2010).
C.-K. Hu, J. Kelly, H. Huang, K. Motoyama, H. Shobha, Y. Ostrovski, J. H.-C. Chen, R. Patlolla, B. Peethala, P. Adusumilli, T. Spooner, R. Quon, L. M. Gignac, C. Breslin, G. Lian, M. Ali, J. Benedict, X. S. Lin, S. Smith, V. Kamineni, X. Zhang, F. Mont, S. Siddiqui, F. Baumann, Future on-chip interconnect metallization and electromigration, in Proceedings of 2018 IEEE International Reliability Physics Symposium (IRPS), 4F1.1–1.6 (2018)
S.H. Hsieh, W.J. Chen, and C.M. Chien, Structural stability of diffusion barriers in Cu/Ru/MgO/Ta/Si. Nanomaterials 5(4), 1840 (2015).
C.-K. Hu, Electromigration and resistivity in on-chip Cu, Co and Ru damascene nanowires, in Proceedings 2017 IEEE International Interconnect Technology Conference (2017).
J.M. Purswani and D. Gall, Electron scattering at single crystal Cu surfaces. Thin Solid Films 516, 465 (2007).
D. Gall, Electron mean free path in elemental metals. J. Appl. Phys. 119, 085101 (2016).
S.-H. Kim, H.T. Kim, S.-S. Yim, D.-J. Lee, K.-S. Kim, H.-M. Kim, K.-B. Kim, and H. Sohn, A bilayer diffusion barrier of ALD-Ru/ALD-TaCN for direct plating of Cu. J. Electrochem. Soc. 155, H589 (2008).
S.-H. Choi, T. Cheon, S.-H. Kim, D.-H. Kang, G.-S. Park, and S. Kim, Thermal atomic layer deposition (ALD) of Ru films for Cu direct plating. J. Electrochem. Soc. 158, D351 (2011).
Y.S. Chan and M.L. Chou, Microstructure evolution of newly developed electroless ruthenium deposition on silicon observed by scanning transmission electron microscope. J. Appl. Phys. 69, 7848 (1991).
J.-Y. Chen, S.-L. Huang, P.-W. Wu, and P. Lin, Electroless deposition of Ru films on Si substrates with surface pretreatments. Thin Solid Films 529, 426 (2011).
J.-Y. Chen, Y.C. Hsieh, L.-Y. Wang, and P.-W. Wu, Electroless deposition of Ru films via an oxidative-reductive mechanism. J. Electrochem. Soc. 158, D463 (2011).
W.J. Dressick, L.M. Kondracki, M.S. Chen, S.L. Brandow, E. Matijevic, and J.M. Calvert, Characterization of a colloidal Pd(II)-based catalyst dispersion for electroless metal deposition. Colloids Surf. A 108–1, 101 (1996).
P. Scherrer, Bestimmung der größe und der inneren struktur von kolloidteilchen mittels röntgenstrahlen. Göttinger Nach richten Math. Phys. 2, 98 (1918).
K. He, N. Chen, C. Wang, L. Wei, and J. Chen, Method for determining crystal grain size by x-ray diffraction. Crst. Res. Technol. 53(2), 1700157 (2018).
J.J. Kim and S.-K. Kim, Optimized surface pretreatments for copper electroplating. Appl. Surf. Sci. 183(3–4), 31 (2001).
K.H. Kim, T. Lim, K.J. Park, H.C. Koo, M.J. Kim, and J.J. Kim, Investigation of Cu growth phenomena on Ru substrate during electroless deposition using hydrazine as a reducing agent. Electrochim. Acta. 151, 249 (2015).
J. Paul Chen and L.L. Lim, Key factors in chemical reduction by hydrazine for recovery of precious metals. Chemosphere 49, 363 (2002).
S.S. Djokić, Electroless deposition of cobalt using hydrazine as a reducing agent. J. Electrochem. Soc. 144(7), 2358 (1997).
J. Kelly, V. Kamineni, X. Lin, A. Pacquette, M. Hopstaken, Y. Liang, H. Amanapu, B. Peethala, L. Jiang, H. Shobha, M. Aymond, and B. Haran, Annealing and impurity effects in Co thin films for MOL contact and BEOL metallization. J. Electrochem. Soc. 166(1), D3100 (2018).
S.Y. Li, J.A. Rodriguez, J. Hrbek, H.H. Huang, and G.Q. Xu, Reaction of hydrogen with O/Ru (001) and RuOx films: formation of hydroxyl and water. J. Vac. Sci. Technol. A15, 1692 (1997).
K. Fuchs, Conduction electrons in thin metallic films, in Proceedings of the Cambridge Philosophical Society, vol. 34 (1938)
E.H. Sondheimer, The mean free path of electrons in metals. Adv. Phys. 50(6), 499 (2001).
H.D. Liu, Y.P. Zhao, G. Ramanath, S.P. Murarka, and G.C. Wang, Thickness dependent electrical resistivity of ultrathin (< 40 nm) Cu films. Thin Solid Films 384(1), 151 (2001).
K. Barmak, A. Gungor, C. Cabral Jr., and J.M.E. Harper, Annealing behavior of Cu and dilute Cu-alloy films: precipitation, grain growth, and resistivity. J. Appl. Phys. 94(3), 1605 (2003).
N. Fukumuro, Y. Fukai, H. Sugimoto, Y. Ishii, H. Saitoh, and S. Yae, Superstoichiometric hydride PdHx ≤ 2 formed by electrochemical synthesis: dissolution as molecular H2 proposed. J. Alloys Compd. 825, 153830 (2020).
I. Koiwa, K. Deguchi, Y. Haijima, N. Hirashita, and K. Maejima, Analyses of plated films by thermal desorption spectrometry (TDS). Surf. Eng. 28–9, 674 (2012).
Z. Wang, O. Yaegashi, H. Sakaue, T. Takahagi, and S. Shingubara, Bottom-up fill for submicrometer copper via holes of ULSIs by electroless plating. J. Electrochem. Soc. 151(12), C781 (2004).
S. Shingubara, Z. Wang, S. Yaegashi, R. Obata, H. Sakaue, and T. Takahagi, Bottom-up fill of Cu in deep submicron holes by electroless plating. Electrochem. Solid State Lett. 7, C78 (2004).
Acknowledgments
The authors are grateful to Mr Y. Morita for his great contribution in the early stage of this study, and to the High Voltage TEM Research Center of Osaka University. We thank Adam Brotchie, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saida, R., Shimizu, T., Ito, T. et al. Electroless Plating of Ru Using Hydrazine Hydrate as a Reducing Agent. J. Electron. Mater. 52, 6690–6698 (2023). https://doi.org/10.1007/s11664-023-10605-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10605-5