Abstract
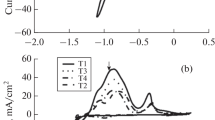
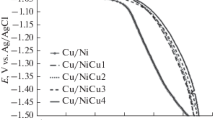
Electrodeposition of metals and alloys from aqueous solutions has been extensively studied in the literature. Recently, ionic liquids having only anions and cations (without solvent) have been used as electrodeposition baths of metals and alloys. Growth conditions may significantly affect the surface characteristics of coatings. In this study, Zn metal, Co metal and Zn-Co alloy-based electrodes were prepared on copper surfaces from ionic liquids solution consisting of stoichiometric 1:2 mixtures of choline chloride and ethylene glycol (Ethaline). The growth potentials of Zn (zinc), Co (cobalt) and Zn-Co (zinc-cobalt alloy) electrodes were analysed by cyclic voltammograms. Zn, Co and Zn-Co were electrodeposited independently on copper substrates using constant voltages and ionic liquid electrolytes containing the salt forms of these metals. The surface morphologies, compositions and structures of the synthesized coatings were investigated by scanning electron microscope, Fourier Transform Infrared spectrophotometer, X-ray Photoelectron Spectroscopy, Energy-Dispersive X-ray Spectroscopy and X-ray Diffractometer. The coatings made of cobalt and zinc contained nanoflakes and hexagonal nano-layers, respectively. The smooth copper surfaces were evenly coated by these nanostructured thin film coatings. The nano and micro sized Co-Zn alloy particles electrodeposited from ionic liquid were observed to be agglomerated. The corrosion behaviour of Zn, Co and Zn-Co alloys was measured in 3.5% NaCl environment, using the Tafel method. The electrodeposition of the Zn-Co alloy resulted in higher corrosion resistances. While the Icorr (corrosion current) value of bare copper electrode was 40.7 μA cm−2, the Icorr value of Zn-Co electrochemically coated on copper was determined as 1.26 μA cm−2. These findings indicated that the corrosion resistivity of the Co-Zn alloy coating was more than 30 times greater than that of uncoated copper.






Similar content being viewed by others
References
Y. Lei, W. Xiao, H. Peng, P. Yu, X. Cai, Z. Luan, S. Deng, and S. Wang, J. Alloys Compd. 853, 157005 (2021).
E.E. Ikechukwu and E.O. Pauline, Open J. Soc. Sci. 3, 143 (2015).
D. Tolentino and C.A. Carrillo-Bueno, KSCE J. Civ. Eng.. 22, 1344 (2018).
X. Li, D. Zhang, Z. Liu, Z. Li, C. Du, and C. Dong, Nat. News 527, 441 (2015).
Y. Zeng, Z. Qin, Q. Hua, Y. Min, and Q. Xu, Surf. Coatings Technol. 362, 62 (2019).
N.H. Othman, M.C. Ismail, M. Mustapha, N. Sallih, K.E. Kee, and R.A. Jaal, Prog. Org. Coatings 135, 82 (2019).
M. Taghavikish, N. K. Dutta, and N. Roy Choudhury, Coatings 7, 217 (2017).
W. Zhan, Q. He, X. Liu, Y. Guo, Y. Wang, L. Wang, Y. Guo, A.Y. Borisevich, J. Zhang, and G. Lu, J. Am. Chem. Soc. 138, 16130 (2016).
Y.J. Cho, H. Jang, K.-S. Lee, and D.R. Kim, Appl. Surf. Sci. 340, 96 (2015).
J. Li, P. Zhang, H. He, and B. Shi, Mater. Des. 187, 108373 (2020).
H. Tian, Z. Guo, J. Pan, D. Zhu, C. Yang, Y. Xue, S. Li, and D. Wang, Resour. Conserv. Recycl. 168, 105366 (2021).
N. Aslan, B. Ceylan, M.M. Koç, and F. Findik, J. Mol. Struct. 1219, 128599 (2020).
J. Zhang and H. Li, Int. J. Electrochem. Sci. 15, 4368 (2020)
D. Ji, X. Wen, T. Foller, Y. You, F. Wang, and R. Joshi, Nanomaterials 10, 2511 (2020).
S. Singh, P. Singh, H. Singh, and R.K. Buddu, Mater. Today Proc. 18, 830 (2019).
J. Balaji, T.H. Oh, and M.G. Sethuraman, J. Taiwan Inst. Chem. Eng. 119, 259 (2021).
T. Ge, W. Zhao, X. Wu, Y. Wu, L. Shen, X. Ci, and Y. He, Mater. Des. 186, 108299 (2020).
H. Huang and X. Guo, Colloids Surfaces A Physicochem. Eng. Asp. 598, 124809 (2020)
G. He, S. Lu, W. Xu, S. Szunerits, R. Boukherroub, and H. Zhang, Phys. Chem. Chem. Phys. 17, 10871 (2015).
Z. Liu, S.Z. El Abedin, and F. Endres, Electrochim. Acta 89, 635 (2013).
R.M. Krishnan, S.R. Natarajan, V.S. Muralidharan, and G. Singh, Plat. Surf. Finish. 79, 67 (1992).
P.P. Chung, P.A. Cantwell, G.D. Wilcox, and G.W. Critchlow, Trans. IMF 86, 211 (2008).
A.Y.M. Al-Murshedi, A. Al-Yasari, H.F. Alesary, and H.K. Ismail, Chem. Pap. 74, 699 (2020).
D.H. Zaitsau, G.J. Kabo, A.A. Strechan, Y.U. Paulechka, A. Tschersich, S.P. Verevkin, and A. Heintz, J. Phys. Chem. A 110, 7303 (2006)
F. Wu, N. Zhu, Y. Bai, L. Liu, H. Zhou, and C. Wu, ACS Appl. Mater. Interfaces 8, 21381 (2016).
T. Boiadjieva, M. Monev, A. Tomandl, H. Kronberger, and G. Fafilek, J. Solid State Electrochem. 13, 671 (2009).
A. Yavuz, N. Ozdemir, P.Y. Erdogan, H. Zengin, G. Zengin, and M. Bedir, Thin Solid Films 711, 138309 (2020).
S.I. Abu-Eishah, Ion. Liq. Prop. 239 (2011).
K.K. Maniam and S. Paul, Appl. Sci. 10, 5321 (2020).
L. Tahraoui, M. Diafi, and A. Fadel, Dig. J. Nanomater. Biostructures 16, (2021).
S. Arora and C. Srivastava, Metall. Mater. Trans. A 51, 4274 (2020).
S. Basavanna and Y.A. Naik, (2012).
T. Nguyen, M. Boudard, M.J. Carmezim, and M.F. Montemor, Sci. Rep. 7, 39980 (2017).
X. Qin, D. Shi, B. Guo, C. Fu, J. Zhang, Q. Xie, X. Shi, F. Chen, X. Qin, and W. Yu, Nanoscale Res. Lett. 15, 1 (2020).
M. Diafi, T. Louiza and K. Digheche, Acta Metall. Slovaca 24, 241 (2018).
F. Zhang, C. Yuan, X. Lu, L. Zhang, Q. Che, and X. Zhang, J. Power Sources 203, 250 (2012).
B.V.A. Rao and M.N. Reddy, Arab. J. Chem. 10, S3270 (2017).
K.M. Pondman, A.W. Maijenburg, F.B. Celikkol, A.A. Pathan, U. Kishore, B. Ten Haken, and E. ten Johan, J. Mater. Chem. B 1, 6129 (2013).
K. Vinothkumar and M.G. Sethuraman, Polym. Bull. 78, 15 (2021).
B. Han, Q. Yan, Z. Xin, Q. Liu, D. Li, J. Wang, and G. He, New J. Chem. 45, 13262 (2021).
M. Verma, L. Sinha, and P.M. Shirage, J. Mater. Sci. Mater. Electron. 32, 12292 (2021).
Q. Qian, F. Wang, X. Zhang, and Q. Zhao, Inorg. Chem. Commun. 127, 108555 (2021).
D. Xu, D. Fan, and W.Shen, Nanoscale Res. Lett. 8, 1 (2013).
D.V. Ponnuvelu, S. Abdulla, and B. Pullithadathil, ChemistrySelect 3, 7156 (2018).
Acknowledgments
PYE thanks the Council of Higher Education (YOK) in Turkey for 100/2000 PhD scholarship. This work was supported by Gaziantep University, BAP (FEF.DT.19.40).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yavuz, A., Yilmaz Erdogan, P., Zengin, H. et al. Electrodeposition and Characterisation of Zn-Co Alloys from Ionic Liquids on Copper. J. Electron. Mater. 51, 5253–5261 (2022). https://doi.org/10.1007/s11664-022-09756-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09756-8