Abstract
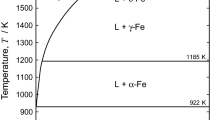
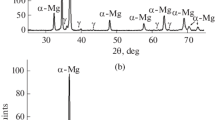
There is a necessity for quantitative information on the solubilities of various elements in Mg and its alloys owing to the growing interest in the precise control of their compositions. In this context, we investigated the quantitative dissolution of metallic Cr in liquid Mg. Pure Mg was melted in a Cr crucible at the temperature range of approximately 1073 K to 1323 K (800 °C to 1050 °C), and the composition of Mg was valuated after quenching. As a result, the relationship between the solubility limit of Cr in liquid Mg (Csol,Cr (mass pct)) and temperature (T/K) was found to be
Furthermore, the thermodynamic parameters for the Cr dissolution were assessed based on the experimental solubility data. The standard Gibbs energy of Cr dissolution in liquid Mg (ΔG°1,Cr/J) was evaluated to be as follows:
Cr(s) = Cr(1 mass pct, in liquid Mg)
ΔG°1,Cr = − 68.5 × T + 98400 (±1500).
The findings in this study are beneficial for refining the Cr-Mg phase diagram and improving the impurity control of Mg in various industries such as Mg-alloy production and Ti smelting.







Similar content being viewed by others
References
Roskill Information Services: Magnesium Metal Outlook to 2030, 13th ed. Roskill Information Services Ltd., London, UK, 2020.
H. Okamoto: Phase Equilib., 2000, vol. 21(2), p. 209.
J. Buha: Mater. Sci. Eng. A., 2008, vol. 492, pp. 293–299. https://doi.org/10.1016/j.msea.2008.04.014.
COST 507, Thermodynamical Database for Light Metal Alloys, I. Ansara, A.T. Dinstale, and M.H. Rand, eds., European Communities, Luxembourg, 1998, vol. 2, pp. 143−144.
Y. Taninouchi and T.H. Okabe: Metall. Mater. Trans. B., 2021, vol. 52B, pp. 611–24. https://doi.org/10.1007/s11663-020-02025-1.
X. Huang, Y. Ma, Q. Zhang, L. Wei, and J. Yang: China Foundry., 2019, vol. 16, pp. 53–62. https://doi.org/10.1007/s41230-019-8112-z.
Magnesium Technology: Metallurgy, Design Data, Applications H.E. Friedrich and B.L. Mordike, eds., Springer, Berlin, 2006.
F. Czerwinski, Corrosion of Materials in Liquid Magnesium Alloys and Its Prevention, in: F. Czerwinski, ed., Magnesium Alloys - Properties in Solid and Liquid States, IntechOpen, 2014, pp. 131−170
K. Sakakibara: J. JFS., 2014, vol. 86(8), pp. 639–51 (in Japanese).
H. Kusamichi, J. Iseki, A. Moriya, A. Kanai, T. Nishimura, H. Kanayama, and T. Kusamichi: Titanium Industry in Japan and Its New Technologies, AGNE Gijutsu Center, Tokyo, 1996 (in Japanese).
Y. Taninouchi, K. Nose, and T.H. Okabe: Metall. Mater. Trans. B., 2018, vol. 49B, pp. 3432–43. https://doi.org/10.1007/s11663-018-1384-7.
T. Chen, X. Xiong, Y. Yuan, A. Tang, D. Li, A. Atrens, and F. Pan: Adv. Eng. Mater., 2020, https://doi.org/10.1002/adem.202000338.
FactSage Thermochemical Software (version 7.3) and Databases (FactPS). www.factsage.com.
Japanese Industrial Standards; JIS G 4303: 2012.
The SGTE Unary Database, version 5.0. http://www.crct.polymtl.ca/sgte/index.php?free=1
Acknowledgments
The authors are grateful to Messrs. Kazuhiro Taki, Masanori Yamaguchi, Yosuke Inoue, and Meiji Watanabe of Toho Titanium Co., Ltd. for the valuable suggestions and useful information provided by them. The authors also thank Messrs. Satoshi Oue, Eiji Shirane, and Yoji Iwai of Kyushu University for their support on the machining of Cr metal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taninouchi, Yk., Yamaguchi, T., Okabe, T.H. et al. Solubility of Chromium in Liquid Magnesium. Metall Mater Trans B 53, 1851–1857 (2022). https://doi.org/10.1007/s11663-022-02494-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02494-6