Abstract
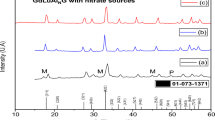
Lithium triborate (LiB3O5) was produced by different synthesis methods, which included high-temperature solid-state reaction, microwave-assisted high-temperature solid-state reaction, and precipitation-assisted high-temperature solid-state reaction. After the synthesis, metal oxides (CuO and Al2O3) were doped into LiB3O5 to enhance its thermoluminescent (TL) properties, and the TL intensities were compared with each other. The identification and characteristics of undoped and doped LiB3O5 were determined by X-ray diffraction (XRD), Fourier transform infrared (FTIR) analyses, differential thermal analyses (DTA), scanning electron microscopy (SEM), and particle size analyzer. The glow curves were obtained by using a TL reader. The results showed that synthesis routes affected the physical and structural properties of lithium triborate, which have an important effect on its TL intensity.












Similar content being viewed by others
References
Z. Özdemir, G. Özbayoğlu, and A. Yılmaz: J. Mater. Sci., 2007, vol. 42, pp. 8501-08.
T. Depci, G. Özbayoğlu, A. Yılmaz, and A.N. Yazici: Nucl. Instrum. Meth. B, 2008, vol. 266, no. 5, pp. 755-62.
V.E. Kafadar, A.N. Yazici, and R.G. Yildirim: J. Lumin., 2009, vol. 129, pp. 710-14.
H. König and R. Hoppe: Z. Anorg. Allg. Chem., 1978, vol. 439, pp. 71-79.
E. Bétourné and M. Touboul: J. Alloys Compd., 1997, vol. 255, pp. 91-97.
S.C. Sabharwal and B. Tiwari, Sangeeta: J. Cryst. Growth, 2003, vol. 249, pp. 502–06.
N.V. Surovtsev, V.K. Malinousky, V.P. Solntsev, A.V. Davydov, and E.G. Tsvetkov: J. Cryst. Growth, 2008, vol. 310, pp. 3540-44.
T. Depci, G. Özbayoğlu, and A. Yilmaz: Physicochem. Probl. Miner. Process., 2007, vol. 41, pp. 101-05.
A. Loupy, Ed.: Microwaves in Organic Synthesis, Wiley-VCH Verlag GmbH and Co., Weinheim, Germany, 2002, pp. 1–73.
I. Ganesh, B. Srinivas, R. Johnson, B.P. Saha, and Y.R. Mahanjan: J. Am. Ceram. Soc., 2004, vol. 24, pp. 201-27.
R.N. Das: Mater. Lett., 2001, vol. 47, pp. 344-50.
P.M. Schaber, J. Colson, S. Higgins, D. Thielen, B. Anspach, and J. Brauer: Thermochim. Acta, 2004, vol. 424, pp. 131-42.
A.M. Garcia, M. Ortiz, R. Martines, P. Ortiz, and E. Reguera: Ind. Crop. Prod., 2004, vol. 19, pp. 99-106.
S. Vanetsev, V.K. Ivanov, and Y.D. Tret’yakov: Dokl. Chem., 2002, vol. 387, pp. 332-34.
U. Moryc and W.S. Ptak: J. Mol. Struct., 1999, vol. 511, pp. 241-49.
L. Xu, B. Wei, Z. Zhang, Z. Lu, H. Gao, and Y. Zhang: Nanatechnology, 2006, vol. 17, pp. 4327-31.
A. Saberi, B. Alinejad, Z. Negahdari, F. Kazemi, and A. Almasi: Mater. Res. Bull., 2007, vol. 42, pp. 666-73.
B. S. R. Sastry and F.A. Hummel: J. Am. Ceram. Soc., 1958, vol. 41, no. 1, pp. 7-17.
M.D. Mathews, A.K. Tyagi, and P.N. Moorthy: Thermochim. Acta, 1998, vol. 320, pp. 89-95.
U.D. Altermatt and I.D. Brown: Acta Cryst., 1987, vol. A34, pp. 125-30.
D. Thomazini, F. Lanciotti, and A.S.B. Sombra: Ceramica, 2001, vol. 47, pp. 88-93.
V. Singh and T.K.G. Rao: J. Solid State Chem., 2008, vol. 181, pp. 1387-92.
S.W.S. McKeever, M. Moskovitch, and P.D. Townsend: Thermoluminescence Dosimetry Materials: Properties and Uses, Nuclear Technologies Publishing, Ashford, UK, 1995.
L. Wei, D. Guiqing, H. Qingzhen, Z. An, and L. Jingkui: J. Phys. D: Appl. Phys., 1990, vol. 23, pp. 1073-75.
Y. F. Shepelev, R.S. Bubnova, S.K. Filatov, N.A. Sennova, and N.A. Pilnev: J. Solid State Chem., 2005, vol. 178, pp. 2987-97.
J. Manam and S.K. Sharma: Nuclear Instrum. Meth. B, 2004, vol. 217, pp. 314-20.
J.K. Srivastava and S.J. Supe: J. Phys. D: Applied Physics, 1989, vol. 22, no. 6, pp. 1537-43.
Y. Kutomi and A. Tomita: Proc. 10th Int. Conf. On Solid State Dosimetry, Washington, DC, 1992, p. 42.
M. Santiago: J. Mater. Sci. Lett., 1998, vol. 17, pp. 1293-96.
M. Ohta and M. Takami: J. Lumin., 2002, vol. 96, pp. 1-4.
C.M.H. Driscoll: Phys. Med. Biol., 1977, vol. 23, pp. 777-81.
C M H. Driscoll and A.F. McKinlay: Phys. Med. Biol., 1981, vol. 26, pp. 321-27.
S. Lorrain, J.P. David, R. Visocekas, and G. Marinello: Radiat. Prot. Dosim., 1986, vol. 17, pp. 385-92.
S.J. Dhoble, S.V. Moharil, S.M. Dhopte, P.L. Muthal, and V.K. Kondawar: Phys. Sol. Stat. A, 1993, vol. 135, pp. 289-98.
K. Shinsho, K. Harada, Y. Yamamoto, and A., Urushiyama: Radiat. Meas., 2008, vol. 43, pp. 236-40.
Acknowledgment
The financial support from the OYP (Scientific HR Development Program) program (BAP-08-11-DPT2002120510) and BOREN (National Boron Research Institute) (2006-05-611-05) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted June 27, 2009.
Rights and permissions
About this article
Cite this article
Depci, T., Ozbayoglu, G. & Yilmaz, A. Comparison of Different Synthesis Methods to Produce Lithium Triborate and Their Effects on Its Thermoluminescent Property. Metall Mater Trans A 41, 2584–2594 (2010). https://doi.org/10.1007/s11661-010-0341-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-010-0341-0