Abstract
Objective
To analyze clinical feature and information of medication to explore the risk signals of preparations containing Psoraleae Fructus (BGZP) related with hepatobiliary adverse drug reactions (ADR), in order to reinforce pharmacovigilance.
Methods
A retrospective study was conducted based on hepatobiliary ADR related with BGZP from the China Adverse Drug Reaction Monitoring System in years from January 2012 to December 2016. Serious and general ADRs were analyzed and assessed.
Results
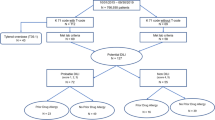
There were 355 cases of hepatobiliary ADR related to BGZP. Both the amount of cases and the proportion of serious ADR showed an increasing growth by years (P<0.05). It was found that 10.43% of 355 cases may be involved with irrational drug use, including overdose, repeated medication, and combination of multiple drugs. There were 190 cases which used BGZP (non-combination), and they were mainly for common in diseases caused by abnormal immune activation (accounting for 40.53% of the total cases). Especially at the age group with the most cases with age of 41–50 years, the cases associated with immunological diseases of female were obviously more than that of male (P<0.05). The latency of hepatobiliary ADR related to BGZP ranged from 1 to 386 days, and the median latency was 27.5 days, along with the range of cumulative dose (0.45–520.02 g) as well as the daily dose (0.09–2.64 g/d) after the conversion.
Conclusions
Cases of hepatobiliary ADR related to BGZP showed significant individual differences, and there was no correlation between drug usage duration and dosage and the occurrence of hepatobiliary ADR. It may be similar with idiosyncratic drug-induced liver injury, and recommended that BGZP should be used with more caution under monitoring liver function, especially in female patients with immunological diseases.
Similar content being viewed by others
References
Yang JJ, Yang J, Du J, Feng YX, Chai X, Xiao MM, et al. General survey of Fructus Psoraleae from the different origins and chemical identification of the roasted from raw Fructus Psoraleae. J Food Drug Anal 2017;26:1–8.
Wang JY, Jiang ZZ, Ji JZ, Li YY, Chen M, Wang YR, et al. Evaluation of hepatotoxicity and cholestasis in rats treated with EtOH extract of Fructus Psoraleae. J Ethnopharmacology 2012;144:73–81.
National Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China. Beijing: Chinese Medical Science and Technology Press 2015;1:188.
Garg BJ, Garg NK, Beg S, Singh B, Katare OP. Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: formulation optimization, in vitro evaluation and preclinical assessment. J Drug Target 2016;24:233–246.
Huang FF, Liang SM, Wang LY, Yang DP. Study of the pharmacodynamics of psoralen gel on experimental vitiligo. Chin J Hosp Pharm (Chin) 2007;27:36–38.
Zhou PL, Ouyang H, Wang ZX. The advancement of drug therapy for vitiligo. Guid J Tradit Chin Med Pharm (Chin) 2005;11:77–79.
Cao JY, Liu JJ, Huang WH, Guo BL. Progress in the pharmacological action and clinical application of psoralen. Chin Med Pharm Clin (Chin) 2008;24:89–92.
Wang W, Liao SP, Wei L. Clinical efficacy of appendix treatment with Gukang capsules in elder patients with a distal radius fracture. J Chin Med Mater (Chin) 2015;38:193–196.
Qiao CF, Han QB, Song JZ, Mo SF, Kong LD, Kung SF, et al. Chemical fingerprint and quantitative analysis of Fructus Psoraleae by high-performance liquid chromatography. J Sep Sci 2007;30:813–818.
Li WD, Yan CP, Wu Y, Weng ZB, Yin FZ, Yang GM, et al. Osteoblasts proliferation and differentiation stimulating activities of the main components of Fructus Psoraleae corylifoliae. Phytomedicine 2014;21:400–405.
Song HB. Risk signals and main risk characteristics of clinical psoralen drugs based on adverse reaction monitoring data. Chinese Society of Toxicology and Hubei Association for Science & Technology. Proceedings of the 7th National Toxicology Conference of the Chinese Society of Toxicology and the 8th Hubei Science and Technology Forum, Chinese Society of Toxicology and Hubei Association for Science & Technology. Wuhan; 2015.
Nam SW, Baek JT, Lee DS, Kang SB, Ahn BM, Chung KW. A case of acute cholestatic hepatitis associated with the seeds of Psoralea corylifolia (Boh-Gol-Zhee). Clin Tox 2005;43:589–591.
Tian WY, Lan S, Zhang L, Sun L, Huang JK, Yang XH, et al. Safety evaluation and risk control measures of Psoralea corylifolia. Chin a J Chin Mater Med (Chin) 2017;42:4059–4066.
China Food and Drug Administration. Be alert to the risk of liver damage caused by the oral preparation of Xianling Gubao. Shanghai Med (Chin) 2017;38:80.
Jin R, Gu HY, Li LL, Sun LL. Current status of Chinese herbal preparations included in the Liver Tox database. Chin J Hepatol (Chin) 2016;24:817–823.
Li A, Gao MH, Zhao N, Li P, Zhu JG, Li WF. Acute liver failure associated with Fructus Psoraleae: a case report and literature review. BMC Complem Altern M 2019;19:1–4.
World Health Organization (WHO), Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment. Available at: http://www.who-umc.org/Graphics/26649.pdf 2018.
Drug-induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Diagnosis and treatment guideline on drug-induced liver injury. Chin J Hepatol (Chin) 2015;23:810–820.
Wang JB, Zhu Y, Bai ZF, Wang FS, Li XH, Xiao XH. Guidelines for the diagnosis and management of herb-induced liver injury. Chin J Integr Med 2018;24:696–706.
China Food and Drug Administration. Measures for the reporting and monitoring of adverse drug reactions. Available at: http://samr.cfda.gov.cn/WS01/CL1031/62621_7.html. Accessed 04 May 2011.
Gao Y, Wang ZL, Tang JF, Liu XF, Shi W, Qin N, et al. New incompatible pair of TCM: Epimedii Folium combined with Psoraleae Fructus induces idiosyncratic hepatotoxicity under immunological stress conditions. Front Med (Chin) 2020;14:68–80.
Tang JF, Li WX, Wang XY, Li YH, Zhang HZ, Cao YJ, et al. Related biomarker screening of Zhuanggu Guanjie Pill induced idiosyncrasy liver injury based on metabolomics. China J Trait Chin Med Pharm (Chin) 2018;33:4336–4340.
Tang JF, Wang XY, Yang W, Li WX, Li YH, Bai ZF, et al. Cytokine analysis of Zhuangguguanjie wan-induced idiosyncratic liver injury based on mathematical modeling. Acta Pharm Sin 2018;53:574–584.
Ge FL, Niu M, Han ZX, Zhang YF, Wang JB, Xiao XH, et al. Analysis of epidemiological characteristics of drug induced liver injury associated with Baixianpi preparations. China J Chin Mater Med (Chin) 2019;44:1048–1052.
Xia LT, Rang C, Wang DY. Analysis on the standardization and rationality of the prescription of Chinese herbal decoction pieces. Chin J Clin Ration Drug Use (Chin) 2019;12:77–78.
Shen T, Liu YX, Shang J, Xie Q, Li J, Yan M, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology 2019;156:2230–2241.
Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. Gastroenterology 1999;118:984–985.
O,Dell JR, Zetterman RK, Burnett DA. Centrilobular hepatic fibrosis following acetaminophen-induced hepatic necrosis in an alcoholic. JAMA 1986;255:2636.
Author information
Authors and Affiliations
Contributions
Ge FL carried out the protocol and drafted the manuscript. Niu M and Han ZX carried out the acquisition of data and analysis. Cao JL and Bai ZF participated in the data extracting and quality control. Wang JB and Xiao XH gave good advice and revised the manuscript. Song HB and Guo YM have made equal substantial contributions to design and manage this protocol. All authors have read and approved the final version.
Corresponding authors
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Supported by the National Natural Science Foundation of China (No. 81630100), Beijing Nova Program (No. Z181100006218001), and Project of China PLA General Hospital (No. 2019MBD-023)
Supplementary material to
11655_2021_3442_MOESM1_ESM.pdf
Landscape of Hepatobiliary Adverse Drug Reactions Related to Preparations Containing Psoraleae Fructus and Its Application in Pharmacovigilance
Rights and permissions
About this article
Cite this article
Ge, Fl., Niu, M., Han, Zx. et al. Landscape of Hepatobiliary Adverse Drug Reactions Related to Preparations Containing Psoraleae Fructus and Its Application in Pharmacovigilance. Chin. J. Integr. Med. 27, 832–837 (2021). https://doi.org/10.1007/s11655-021-3442-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-021-3442-2