Abstract
Background
Periostin (POSTN) is involved in many biological processes and is associated with the occurrence and development of several cancers, while its role in gastric cancer is not clear. We aimed to demonstrate the relationship between POSTN and gastric cancer based on publicly available data from The Cancer Genome Atlas (TCGA) database.
Methods
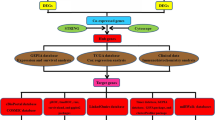
POSTN expression data and corresponding clinical information were downloaded from TCGA database. We compared the expression of POSTN in gastric cancer samples and normal samples. POSTN-related differentially expressed genes (DEGs) were identified for further functional enrichment analysis. In addition, the relationships between POSTN expression and clinicopathological features and survival in patients with gastric cancer were also investigated through univariate and multivariate Cox regression analyses. Furthermore, a nomogram was constructed to predict the survival probability of gastric cancer patients.
Results
POSTN expression in gastric cancer was significantly higher than that in normal gastric tissues (p < 0.001). High POSTN expression in gastric cancer was significantly related to poor prognostic features, including greater tumor extent (odds ratio [OR] = 1.638 for T3 and T4 vs. T1 and T2), worse histological type (OR = 0.329 for diffuse type vs. tubular type), and advanced histological grade (OR = 1.646 for grade 3 vs. grades 1 and 2) (all p < 0.05). The 118 identified DEGs were primarily enriched in pathways related to tumorigenesis and tumor progression, including the TGF-β signaling pathway, the WNT signaling pathway, and the signaling by VEGF. POSTN expression was positively correlated with the enrichment of the macrophages (r = 0.599, p < 0.001), helper T2 cells (r = 0.146, p = 0.005), and CD8 + T cells (r = 0.190, p < 0.001), but negatively correlated with the enrichment of Th17 cells (r = − 0.130, p = 0.012) and NK CD56bright cells. Kaplan–Meier survival analysis showed that high POSTN expression is associated with a short overall survival (hazard ratio [HR] = 1.54; p = 0.011). In the multivariate cox regression analysis, high POSTN expression was confirmed to be an independent predictor of poor overall survival (HR = 1.681; p = 0.017). The constructed nomogram can well predict the 1 and 3 years overall survival probability of patients with GC (0.696 [95% CI, 0.671–0.721]).
Conclusion
POSTN plays an important role in the progression and prognosis of gastric cancer, and it may serve as a useful biomarker to predict survival in gastric cancer patients.







Similar content being viewed by others
References
Sung H, Ferlay J, Rebecca L, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin 2021;0:1–41. https://doi.org/10.3322/caac.21660.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. https://doi.org/10.1016/S0140-6736(17)33326-3
Winkler J, Abisoye-Ogunniyan A, Metcalf K, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020 Oct 9;11(1):5120. https://doi.org/10.1038/s41467-020-18794-x
Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr H, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature (2011) 481, 85–89. https://doi.org/10.1038/nature10694
Ratajczak-Wielgomas K, Kmiecik A, Dziegiel P. Role of Periostin Expression in Non-Small Cell Lung Cancer: Periostin Silencing Inhibits the Migration and Invasion of Lung Cancer Cells via Regulation of MMP-2 Expression. Int J Mol Sci. 2022 22;23(3):1240. https://doi.org/10.3390/ijms23031240.
Ma Y, He L, Zhao X, Li W, Lv X, et al. Protease activated receptor 2 signaling promotes self-renewal and metastasis in colorectal cancer through β-catenin and periostin. Cancer Lett. 2021 27;521:130-141. https://doi.org/10.1016/j.canlet.2021.08.032
Yu B, Wu K, Wang X, Zhang J, Wang L, et al. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 2018 22;9(11):1082. https://doi.org/10.1038/s41419-018-1116-6.
Underwood T, Hayden A, Derouet M, Garcia E, Noble F, et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol. 2015; 235(3):466-77. https://doi.org/10.1002/path.4467
Lv Y, Wang W, Jia W, Sun Q, Huang M, et al. High preoparative levels of serum periostin are associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Eur J Surg Oncol. 2013 Oct;39(10):1129-35. https://doi.org/10.1016/j.ejso.2013.06.023
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. (2014) 15:550. https://doi.org/10.1186/s13059-014-0550-8
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. (2012) 16:284–7. https://doi.org/10.1089/omi.2011.0118
Subramanian A, Tamayo P, Mootha V K, Mukherjee S, Ebert B, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A, 2005, 102(43): 15545-15550. https://doi.org/10.1073/pnas.0506580102
Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC bioinformatics 14.1 (2013): 1-15. https://doi.org/10.1186/1471-2105-14-7
Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39.4 (2013): 782-795. https://doi.org/10.1016/j.immuni.2013.10.003
Szklarczyk D, Gable A, Lyon D, Junge A, Wyder S, et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic acids research, 2019, 47(D1): D607-D613. https://doi.org/10.1093/nar/gky1131
Gabor C, Nepusz T. The igraph software package for complex network research. Inter Journal, complex systems 1695.5 (2006): 1–9. https://www.researchgate.net/publication/221995787
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. (2011) 12:77. https://doi.org/10.1186/1471-2105-12-77
Vivian J, Rao AA, Nothaft FA, Ketchum C, Armstrong J, et al. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol. (2017) 35:314-6. https://doi.org/10.1038/nbt.3772
Wong M, Huang J, Chan P, Choi P, Lao X, et al. Global Incidence and Mortality of Gastric Cancer, 1980-2018. JAMA Netw Open. 2021 Jul 1;4(7):e2118457.
Allemani C, Matsuda T, Carlo V, Harewood R, Matz M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018 Mar 17;391(10125):1023-1075. https://doi.org/10.1016/S0140-6736(17)33326-3
Yue H, Li W, Chen R, et al. Stromal POSTN induced by TGF-β1 facilitates the migration and invasion of ovarian cancer. Gynecol Oncol. 2021 Feb;160(2):530-538. https://doi.org/10.1016/j.ygyno.2020.11.026
Ouyang G, Liu M, Ruan K, Song G, Mao Y, et al. Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB pathway. Cancer Lett. 2009 28;281(2):213–9. https://doi.org/10.1016/j.canlet.2009.02.030
Kim G, Lee J, Park M, Yoon J. Epithelial periostin expression is correlated with poor survival in patients with invasive breast carcinoma. PLoS One. 2017 Nov 21;12(11):e0187635. https://doi.org/10.1371/journal.pone.0187635
Labrèche C, Cook D, Abou-Hamad J, et al. Periostin gene expression in neu-positive breast cancer cells is regulated by a FGFR signaling cross talk with TGFβ/PI3K/AKT pathways. Breast Cancer Res. 2021 Nov 22;23(1):107. https://doi.org/10.1186/s13058-021-01487-8
Bowen D, Joong S. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules, 2016, 21(7):965. https://doi.org/10.3390/molecules21070965
Massague J. TGFβ in cancer. Cell. 2008;134(2):215-230. https://doi.org/10.1016/j.cell.2008.07.001
Batlle E, Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50(4):924-940. https://doi.org/10.1016/j.immuni.2019.03.024
Yan Y, Zhang J, Li JH, et al. High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial-mesenchymal transition in gastric cancer. OncoTargets and Therapy, 2016(9): 3975-3983. https://doi.org/10.2147/OTT.S103112
Herr P, Hausmann G, Basler K. WNT secretion and signalling in human disease. Trends Mol Med, 2012;18(8):483-93. https://doi.org/10.1016/j.molmed.2012.06.008
Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–99. https://doi.org/10.1016/j.cell.2017.05.016
Wang Y, Zheng L, Shang W, Yang Z, Li T, et al. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022. https://doi.org/10.1038/s41418-022-01008-w
Pang L, Wang J, Fan Y, Xu R, Bai Y, et al. Correlations of TNM staging and lymph node metastasis of gastric cancer with MRI features and VEGF expression. Cancer Biomark. 2018;23(1):53-59. https://doi.org/10.3233/CBM-181287
Yan R, Peng X, Yuan X, et al. Suppression of growth and migration by blocking the Hedgehog signaling pathway in gastric cancer cells. Cell Oncol, 2013, 36(5):421-435. https://doi.org/10.1007/s13402-013-0149-1
Murakami D, Tsujitani S, Osaki T, Saito H, Katano K, et al. Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy. Gastric Cancer. 2007;10(1):45-51. https://doi.org/10.1007/s10120-006-0410-7
Hu Y, Zheng M, Zhang R, Liang Y, Han H. Notch Signaling Pathway and Cancer Metastasis. Adv Exp Med Biol. 2012, 727, 186–198. https://doi.org/10.1007/978-1-4614-0899-4_14
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002). https://doi.org/10.1016/s1471-4906(02)02302-5
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 454, 436–444. https://doi.org/10.1038/nature07205
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141(1):39-51. https://doi.org/10.1016/j.cell.2010.03.014.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41(1):49-61. https://doi.org/10.1016/j.immuni.2014.06.010.
Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, et al. Evaluations of interferon-γ/interleuki‑4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010 1;102(7):742–7. https://doi.org/10.1002/jso.21725
Chen J, Xia J, Liang X, Pan K, Wang W, et al. Intratumoral expression of IL‑17 and its prognostic role in gastric adenocarcinoma patients. Int. J. Biol. Sci. 7, 53–60 (2011). Int J Biol Sci. 2011 Jan 11;7(1):53–60. https://doi.org/10.7150/ijbs.7.53
Acknowledgements
The authors would like to thank Dr. Wei Yang for his assistance in data acquisition and guidance on the design of this study.
Funding
This study was supported by the Tackle Key Problems in Medicine of Henan Province (201003124, LHGJ20200188).
Author information
Authors and Affiliations
Contributions
Concept and design: SL. Acquisition, analysis, or interpretation of data: SL, WY, LP, and FM. Drafting of the manuscript: SL and WY. Critical revision of the manuscript for important intellectual content: ZZ and YH. Administrative, technical, or material support, and study supervision: ZZ and YH. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Consent to Participate
All data used in this research were obtained from TCGA, a publicly available cancer database, so according to Swedish law, this study was exempt from the need to obtain signed informed consent.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Data Sheet 1
(XLSX 24.1 KB)
Supplementary Data Sheet 2
(XLSX 17.8 KB)
Supplementary Data Sheet 3
(XLSX 781 KB)
Supplementary Table 1
(DOCX 18 KB)
Supplementary Table 2
(DOCX 18 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, S., Peng, L., Ma, F. et al. Increased Expression of POSTN Predicts Poor Prognosis: a Potential Therapeutic Target for Gastric Cancer. J Gastrointest Surg 27, 233–249 (2023). https://doi.org/10.1007/s11605-022-05517-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05517-4