Abstract
Objectives
Approximately 20–40% of patients with locally advanced esophageal cancer will achieve a pathologic complete response (ypCR) following neoadjuvant chemoradiotherapy (nCRT). Predicting ypCR based on a clinical complete response (ycCR) has been a challenge. This study assessed the correlation between ycCR and ypCR, as determined from esophagectomy specimens.
Methods
Patients undergoing esophagectomy following nCRT at three major institutions between 2005 and 2018 were reviewed. Restaging, including PET/CT, endoscopy with biopsy, and esophageal ultrasound (EUS), was performed to determine ycCR.
Results
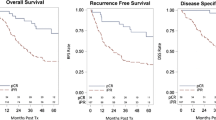
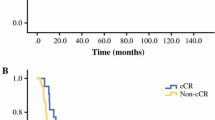
Six hundred sixty patients were included, with 93.3% with esophageal adenocarcinoma histology. Six hundred fifty-eight of these patients underwent PET, 304 EUS, and 584 underwent a biopsy. Following nCRT, 148 (22.4%) were found to have a ypCR. Only 12/32 (37.5%) determined to have a ycCR were found to have a ypCR, while 136/628 (21.6%) with a non-ycCR were found to have a ypCR (p 0.075). Individual modality PPV was 28% for PET, 54% for EUS, and 26% for biopsy. When PET was combined with EUS, 168 reports were concordant and the PPV of ypCR was 50%, though the number of patients was low (1/2). With all 3 re-staging modalities combined, the PPV and NPV both rose to 100%.
Conclusions
Current restaging tools cannot reliably predict ypCR after nCRT. While multimodal restaging appears to be a more accurate predictor of ypCR than any testing modality alone, patients cannot reliably be advised to avoid an esophagectomy on the assumption that ycCR predicts ypCR at this time.
Similar content being viewed by others
Abbreviations
- CRT:
-
Chemoradiation therapy (CRT)
- CT:
-
Computed tomography
- EAC:
-
Esophageal adenocarcinoma
- ESCC:
-
Esophageal squamous cell carcinoma
- EUS:
-
Endoscopic ultrasound
- nCRT:
-
Neoadjuvant chemoradiation therapy
- NPV:
-
Negative predictive value
- PET:
-
Positron emission tomography
- PPV:
-
Positive predictive value
- SUV:
-
Standardized uptake value
- ycCR:
-
Clinical complete response
- ypCR:
-
Pathologic complete response
References
Cancer.Net Editorial Board. Esophageal Cancer Statistics, <https://www.cancer.net/cancer-types/esophageal-cancer/statistics> (2021). Accessed 1 Aug 2021.
Blum Murphy, M. et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer 123, 4106-4113, doi:https://doi.org/10.1002/cncr.30953 (2017).
Meredith, K. L. et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 17, 1159-1167, doi:https://doi.org/10.1245/s10434-009-0862-1 (2010).
Shapiro, J. et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16, 1090-1098, doi:https://doi.org/10.1016/S1470-2045(15)00040-6 (2015).
Medical Research Council Oesophageal Cancer Working, G. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359, 1727-1733, doi:https://doi.org/10.1016/S0140-6736(02)08651-8 (2002).
Allum, W. H., Stenning, S. P., Bancewicz, J., Clark, P. I. & Langley, R. E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27, 5062-5067, doi:https://doi.org/10.1200/JCO.2009.22.2083 (2009).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355, 11-20, doi:https://doi.org/10.1056/NEJMoa055531 (2006).
Ychou, M. et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29, 1715-1721, doi:https://doi.org/10.1200/JCO.2010.33.0597 (2011).
Homann, N. et al. Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5-fluorouracil, oxaliplatin and docetaxel. Int J Cancer 130, 1706-1713, doi:https://doi.org/10.1002/ijc.26180 (2012).
Lorenzen, S. et al. Feasibility of perioperative chemotherapy with infusional 5-FU, leucovorin, and oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly patients with locally advanced esophagogastric cancer. Br J Cancer 108, 519-526, doi:https://doi.org/10.1038/bjc.2012.588 (2013).
van Hagen, P. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366, 2074-2084, doi:https://doi.org/10.1056/NEJMoa1112088 (2012).
Yang, H. et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 36, 2796-2803, doi:https://doi.org/10.1200/JCO.2018.79.1483 (2018).
Stahl, M. et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 27, 851-856, doi:https://doi.org/10.1200/JCO.2008.17.0506 (2009).
Stahl, M. et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer 81, 183-190, doi:https://doi.org/10.1016/j.ejca.2017.04.027 (2017).
de Gouw, D. et al. Detecting Pathological Complete Response in Esophageal Cancer after Neoadjuvant Therapy Based on Imaging Techniques: A Diagnostic Systematic Review and Meta-Analysis. J Thorac Oncol 14, 1156-1171, doi:https://doi.org/10.1016/j.jtho.2019.04.004 (2019).
Kim, M. K. et al. Value of complete metabolic response by (18)F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapy. Eur J Cancer 43, 1385-1391, doi:https://doi.org/10.1016/j.ejca.2007.04.001 (2007).
Cerfolio, R. J., Bryant, A. S., Ohja, B., Bartolucci, A. A. & Eloubeidi, M. A. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg 129, 1232-1241, doi:https://doi.org/10.1016/j.jtcvs.2004.12.042 (2005).
Jones, D. R., Parker, L. A., Jr., Detterbeck, F. C. & Egan, T. M. Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer 85, 1026-1032, doi:https://doi.org/10.1002/(sici)1097-0142(19990301)85:5<1026::aid-cncr3>3.0.co;2-n (1999).
Heethuis, S. E. et al. Dynamic contrast-enhanced MRI for treatment response assessment in patients with oesophageal cancer receiving neoadjuvant chemoradiotherapy. Radiother Oncol 120, 128-135, doi:https://doi.org/10.1016/j.radonc.2016.05.009 (2016).
McLoughlin, J. M. et al. Are patients with esophageal cancer who become PET negative after neoadjuvant chemoradiation free of cancer? J Am Coll Surg 206, 879-886; discussion 886-877, doi:https://doi.org/10.1016/j.jamcollsurg.2007.12.027 (2008).
Cheedella, N. K. et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann Oncol 24, 1262-1266, doi:https://doi.org/10.1093/annonc/mds617 (2013).
Liu, S. L. et al. Is There a Correlation Between Clinical Complete Response and Pathological Complete Response After Neoadjuvant Chemoradiotherapy for Esophageal Squamous Cell Cancer? Ann Surg Oncol 23, 273-281, doi:https://doi.org/10.1245/s10434-015-4764-0 (2016).
Yu, W. et al. GTV spatial conformity between different delineation methods by 18FDG PET/CT and pathology in esophageal cancer. Radiother Oncol 93, 441-446, doi:https://doi.org/10.1016/j.radonc.2009.07.003 (2009).
Vali, F. S. et al. Comparison of standardized uptake value-based positron emission tomography and computed tomography target volumes in esophageal cancer patients undergoing radiotherapy. Int J Radiat Oncol Biol Phys 78, 1057-1063, doi:https://doi.org/10.1016/j.ijrobp.2009.09.022 (2010).
Uberoi, G. S., Uberoi, A. S. & Bhutani, M. S. Endoscopic and Imaging Predictors of Complete Pathologic Response After Chemoradiation for Esophageal Cancer. Curr Gastroenterol Rep 19, 57, doi:https://doi.org/10.1007/s11894-017-0594-5 (2017).
Huang, Y. C. et al. Post-chemoradiotherapy FDG PET with qualitative interpretation criteria for outcome stratification in esophageal squamous cell carcinoma. PLoS One 14, e0210055, doi:https://doi.org/10.1371/journal.pone.0210055 (2019).
Acknowledgements
Medstar Surgical Resident Research Program.
Author information
Authors and Affiliations
Contributions
PGK: project development, data analysis, and manuscript writing. TH: project development, data analysis and collection, and manuscript writing. AC: project development, data analysis and collection, and manuscript editing. WLH: project development, data analysis, and manuscript writing/editing. EMB: project development, data analysis and collection, and manuscript editing. NZ: project development, data analysis and collection, and manuscript editing. SD: project development, data analysis, statistics, and manuscript editing. TJW: project development, data analysis, and manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khaitan, P.G., Holliday, T., Carroll, A. et al. Can Clinical Response Predict Pathologic Response Following Neoadjuvant Chemoradiation for Esophageal Cancer?. J Gastrointest Surg 26, 1345–1351 (2022). https://doi.org/10.1007/s11605-022-05315-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05315-y