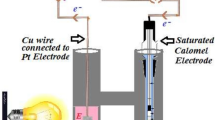
Abstract
The complete electrolyte of photogalvanic cells consists of the sensitizer, reductant, surfactant, alkali, and water solvent. In the present research, the electrochemical properties of the individual chemicals of electrolyte and complete electrolyte (including surfactant) have been studied both in the dark and illuminated conditions. Individual chemical components or combination of any two or three chemical components at low pH show very low potential (≤ 401 mV) and current (≤ 13 µA) under both pre-illuminated (dark) and illuminated conditions. For complete electrolyte (including surfactant) at high pH, the observed dark potential, photo potential, current (in dark), and current (in sunlight) is 495 mV, 720 mV, 522 µA, and 2460 µA, respectively. It has been observed that, in the case of high pH conditions of the complete electrolyte, the abrupt jump in the potential coupled with high photo-current is observed during illumination of the cell. Therefore, it is concluded that high potential and current can be obtained by combining all chemical components of the electrolyte at a time under necessary conditions including the high pH. The photogalvanics is achievable only from complete electrolyte at very high pH. The complete electrolyte (excluding surfactant) at high pH also shows photogalvanics. But, a bit low electrical output for complete electrolyte (excluding surfactant) vis-à-vis that of complete electrolyte (including surfactant) is observed and that may be attributed to the lack of role of surfactant in the dye solubility and stability. It is a reported fact that the surfactant enhances the electrical output of the photogalvanic cells. So by taking all these facts, it may be concluded that the requirement of a surfactant is not a necessary condition for photogalvanics, but its presence in the electrolyte enhances it (photogalvanics). Further, the potential and current of the cell at its initial stage and post-sensitizer degradation stage may be attributed to the polar and ionic nature of the individual chemical components of the electrolyte.








Similar content being viewed by others
Data availability statements
All data generated or analyzed during this study are included in this published article, and its supplementary information file.
References
Lim KG, Kim HB, Jeong J, Kim H, Kim JY, Lee TW (2014) Boosting the power conversion efficiency of perovskite solar cells using self-organized polymeric hole extraction layers with high work function. Adv Mater 26(37):6461–6466
Wohrle D, Meissner D (1991) Organic solar cells. Adv Mater 3(3):129–138
Narayan MR (2012) Review: dye sensitized solar cells based on natural photosensitizers. Renew Sustain Energy Rev 16(1):208–215
Deng Q, Wang X, Yang C, Xiao H, Wang C, Yin H, Hou Q, Li J, Wang Z, Hou X (2011) Theoretical study on InxGa1−xN/GaN quantum dots solar cell. Physica B: Condensed Matter 406(1):73–76
Rabinowitch E (1940) The Photogalvanic effect I, The photochemical properties of the thionine–iron system. J Chem Phys 8(7):551–559
Rabinowitch E (1940) The Photo-galvanic effect II The photochemical properties of the Thionine-iron system. J Chem Phys 8(7):560–566
Koli P (2017) Surfactant and natural sunlight enhanced photogalvanic effect of Sudan I dye. Arab J Chem 10(8):1077–1083
Koli P, Pareek RK, Dayma Y, Jonwal M (2021) Formic acid reductant sodium lauryl sulphate surfactant enhanced photogalvanic effect of indigo carmine dye sensitizer for simultaneous solar energy conversion and storage. Energy Rep 7:3628–3638
Gangotri KM, Solanki PP (2013) Use of sodium lauryl sulphate as a surfactant in a photogalvanic cell for solar energy conversion and storage: a sodium lauryl sulphate-methylene blue-mannose system. Energy Sources, Part A: Recover, Utilization, Environ Eff 35(15):1467–1475
Koli P, Sharma U (2017) Photochemical solar power and storage through photogalvanic cells: comparing performance of dye materials. Energy Sources, Part A: Recovery, Utilization, Environ Eff 39(6):555–561
Koli P (2022) Exploratory insight in to the long term photo-stability of the brilliant cresyl blue based electrolyte solution of photogalvanic cells. J Appl Spectrosc 88(3):361–369
Koli P (2021) U (2021) Sharma Exploratory insight in to stability of rhodamine B and crude aqueous spinach extract based photogalvanic cells: comparing photo-stability of electrolytes for solar power and storage. J Photochem Photobiol 8:100086
Koli P (2014) Solar energy conversion and storage: fast green FCF-fructose photo galvanic cell. Appl Energy 118:231–237
Koli P (2019) Natural sunlight-irradiated Rhodamine B dye sensitized and surfactant-enhanced photo galvanic solar power and storage. Int J Ambient Energy 42(10):1193–1199
Bisquert J, Cahen D, Hodes G, Rühle S, Zaban A (2004) Physical chemical principles of photovoltaic conversion with nano particulate, mesoporous dye-sensitized solar cells. J Phys Chem B 108(24):8106–8118
Enderby JE, Neilson GW (1981) The structure of electrolyte solutions. Rep Prog Phys 44(6):593–653
Koli P, Sharma U, Gangotri KM (2012) Solar energy conversion and storage: rhodamine B - fructose photogalvanic cell. Renew Energy 37:250–258
Murthy ASN, Reddy KS (1983) Studies on photo galvanic effect in systems containing toluidine blue. Sci Direct 30(1):39–43
Lal C (2006) Use of mixed dyes in a photo-galvanic cell for solar energy conversion and storage: EDTA–thionine–azur-B system. J Power Sources 164(2):926–930
Lal M, Gangotri KM (2015) The optimum conversion efficiency in nile blue arabinose system by photo-galvanic cell. Adv Energy Res 3(3):143–155
Sharma U (2010) Use of fructose as reductant in photo-galvanic cell for solar conversion and storage. JNV University, Jodhpur, Rajasthan (India), Thesis
Genwa KR, Sagar CP (2013) Energy efficiency, solar energy conversion and storage in photo-galvanic cell. Energy Convers Manage 66:121–126
Gangotri KM, Bhimwal MK (2011) Study the performance of photo-galvanic cells for solar energy conversion and storage: toluidine blue–D-Xylose–NaLS system. Int J Energy Res 35(6):545–552
Koli P (2017) Photo galvanic effect of natural photosensitizer (crude spinach extract) in artificial light for simultaneous solar power generation and storage. Environ Prog Sustain Energy 37(5):1800–1807
Murthy ASN, Dak HC, Reddy KS (1980) Photogalvanic effect in riboflavin – ethylenediaminetetraacetic acid system. Energy Res 4:339–343
Albery WJ, Foulds AW, Hall KJ, Hillman AR, Egdell RG, Orchard AF (1979) Thionine coated electrode for photogalvanic cells. Nature 282:793–797
Mall C, Solanki PP (2018) Spectrophotometric and conductometric studies of molecular interaction of brilliant cresyl blue with cationic, anionic and non-ionic surfactant in aqueous medium for application in photogalvanic cells for solar energy conversion and storage. Energy Reports 4:23–30
Mahmoud SA, Mohamed BS (2015) Study on the performance of photogalvanic cell for solar energy conversion and storage. Int J Electrochem Sci 10:3340–3353
Lal M, Gangotri KM (2023) Innovative study in renewable energy source through mixed surfactant system for eco-friendly environment. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-28246-w
Koli P, Dayma Y, Pareek RK, Jonwal M (2020) Use of Congo red dye-formaldehyde as a new sensitizer-reductant couple for enhanced simultaneous solar energy conversion and storage by photogalvanic cells at the low and artificial sun intensity. Sci Reps 10:1 (Article number: 19264)
Mahmoud S, Elsisi ME, Mansour AF (2022) Synthesis and electrochemical performance of α-Al2O3 and M-Al2O4 spinel nanocomposites in hybrid quantum dot-sensitized solar cells. Scientific Reports 12:1 (Article number: 17009)
Mahmoud SA, Mohamed BS, Killa HM (2021) Synthesis of different sizes TiO2 and photovoltaic performance in dye-sensitized solar cells. Front Mater 8:714835
Abdelrahman AA, Bendary SH, Mahmoud SA (2023) Synthesis and electrochemical properties of NiAl LDH@RGO hierarchical nanocomposite as a potential counter electrode in dye sensitized solar cells. Diam Relat Mater 134:109738
Bendary SH, Betiha MA, Hussein MF, Mahmoud SA (2022) Solar energy conversion to electricity by Tris (2,2′-bipyirdyl) ruthenium (II) chloride hexahydrate-diethyl ammonium tetrachloroferrate-oxalic acid photogalvanic cell: statistical analysis. J Mol Liq 347:117824
Rohtagi-Mukherjee KK (1986) Fundamentals of photochemistry, New Age international publisher, revised edition, 1986, New Delhi
Tiwari S, Mall C, Solanki PP (2020) CMC studies of CTAB, SLS & tween 80 by spectral and conductivity methodology to explore its potential in photogalvanic cell. Surf Interfaces 18:100427
Acknowledgements
Authors are thankful to the Department of Chemistry, Jai Narain Vyas, University, Jodhpur, Rajasthan (India), for providing all necessary laboratory facilities. Author, Jyoti Saren, is also grateful to the University Grants Commission (India) for providing a fellowship vide UGC JRF Award letter No.: 355/ (CSIR-UGC NET JUNE 2018)/12 April, 2019.
Author information
Authors and Affiliations
Contributions
Authors P.K. and J.S. have contributed to every aspect of this research work including the conception, design of the work, acquisition, analysis, interpretation of data, revision, etc. Authors A.C., A.M., D. and R.K. have contributed to the design of work, participated in experiments, interpretation of data, review of manuscript, English and language checking, etc. Additionally, the author P.K. has also contributed as a corresponding author, mentor, and supervisor of the research work. All authors have approved the submitted version.
Authors P.K. and J.S. have contributed to every aspect of this research work including the conception, design of the work, acquisition, analysis, interpretation of data, and revision. Authors A.C., A.M., D., and R.K. have contributed to the design of work, participated in experiments, interpretation of data, review of manuscript, English and language checking, etc. Additionally, the author P.K. has also contributed as a corresponding author, mentor, and supervisor of the research work. All authors have approved the submitted version.
Corresponding author
Ethics declarations
Competing financial interests
Author has no Competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koli, P., Saren, J., Charan, A. et al. Exploratory insight into the photogalvanics of the complete electrolyte and its individual chemical components. Ionics 30, 1815–1831 (2024). https://doi.org/10.1007/s11581-024-05401-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05401-y