Abstract
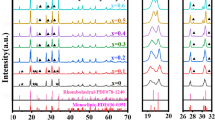
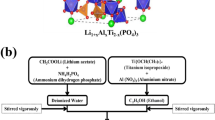
Among many kinds of electrolytes, Li1.3Al0.3Ti1.7(PO4)3 (LATP) ceramic electrolyte has a few focal points such as relatively low price, excellent resistance to air and moisture, high ionic conductivity, and environmental safety. Besides, LATP has a disadvantage in that it exposes batteries to degradation due to the interfacial layer formed after it touches metallic lithium because of the titanium reduction reaction. Herein, we report our results on the strategy of anion doping in LATP structures by Si (LATSP). The LATSP samples with various Si content were synthesized by a simple molten flux method at 800 °C with a pure phase. The structure, morphology, and ion-transport properties were analyzed. It was demonstrated that increasing Si doping content has significant positive effects on electrolyte conductivity.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Jetybayeva A, Uzakbaiuly B, Mukanova A, Myung ST, Bakenov Z (2021) Recent advancements in solid electrolytes integrated into all-solid-state 2D and 3D lithium-ion microbatteries. J Mater Chem A Mater 9:15140–15178. https://doi.org/10.1039/D1TA02652F
Aono H, Sugimoto E, Sadaoka Y, Imanaka N, Adachi G-Y (1990) Ionic conductivity of solid electrolytes based on lithium titanium phosphate. J Electrochem Soc 137:1023. https://doi.org/10.1002/chin.199025008
Arbi K, Rojo JM, Sanz J (2007) Lithium mobility in titanium based Nasicon Li1+xTi2-xAlx(PO4)3 and LiTi2-x Zrx(PO4)3 materials followed by NMR and impedance spectroscopy. J Eur Ceram Soc 27:4215–4218. https://doi.org/10.1016/j.jeurceramsoc.2007.02.118
Lang B, Ziebarth B, Elsässer C (2015) Lithium ion conduction in LiTi2(PO4)3 and related compounds based on the NASICON structure: a first-principles study. Chem Mater 27:5040–5048. https://doi.org/10.1021/acs.chemmater.5b01582
Redhammer GJ, Rettenwander D, Pristat S, Dashjav E, Kumar CMN, Topa D, Tietz F (2016) A single crystal X-ray and powder neutron diffraction study on NASICON-type Li1+xAlxTi2−x(PO4)3 (0≤x≤0.5) crystals: implications on ionic conductivity. Solid State Sci 60:99–107. https://doi.org/10.1016/j.solidstatesciences.2016.08.011
Li J, Liu C, He M, Nie S, Miao C, Sun S, Xu G, Xiao W (2023) Improved the electrochemical performance between ZnO@Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte and lithium metal electrode for all-solid-state lithium-ion batteries. Electrochim Acta 439. https://doi.org/10.1016/j.electacta.2022.141549
Zhu J, Zhao J, Xiang Y, Lin M, Wang H, Zheng B, He H, Wu Q, Huang Y, Yang Y, Huang JY (2020) Chemo-mechanical failure mechanism study in NASICON-type Li1.3Al0.3Ti1.7(PO4)3 solid-state lithium batteries. Chem Mater 32:4998–5008. https://doi.org/10.1021/acs.chemmater.9b05295
Mashekova A, Baltash Y, Yegamkulov M, Trussov I, Bakenov Z, Mukanova A (2022) Polycationic doping of the LATP ceramic electrolyte for Li-ion batteries. RSC Adv 12:29595–29601. https://doi.org/10.1039/D2RA05782D
Wong S, Newman PJ, Best AS, Nairn KM, MacFarlane DR, Forsyth M (1998) Towards elucidating microscopic structural changes in Li-ion conductors Li1+yTi2–yAly[PO4 ]3 and Li1+yTi2–yAly[PO4 ]3–x [MO4 ]x(M=V and Nb): X-ray and27Al and31P NMR studies. J Mater Chem 8:2199–2203. https://doi.org/10.1039/A802752H
Liu M, Li X, Wang X, Yu R, Chen M, Lu Q, Lu B, Shu H, Yang X (2018) Facile synthesis and electrochemical properties of high lithium ionic conductivity Li1.7Al0.3Ti1.7Si0.4P2.6O12 ceramic solid electrolyte. J Alloys Compd 756:103–110. https://doi.org/10.1016/j.jallcom.2018.04.333
Yi E, Wang W, Mohanty S, Kieffer J, Tamaki R, Laine RM (2014) Materials that can replace liquid electrolytes in Li batteries: Superionic conductivities in Li1.7Al0.3Ti1.7Si 0.4P2.6O12. Processing combustion synthesized nanopowders to free standing thin films. J Power Sources 269:577–588. https://doi.org/10.1016/j.jpowsour.2014.07.029
Zhu J, Xiang Y, Zhao J, Wang H, Li Y, Zheng B, He H, Zhang Z, Huang J, Yang Y (2022) Insights into the local structure, microstructure and ionic conductivity of silicon doped NASICON-type solid electrolyte Li1.3Al0.3Ti1.7P3O12. Energy Storage Mater 44:190–196. https://doi.org/10.1016/j.ensm.2021.10.003
Kotobuki M, Koishi M (2013) Preparation of Li1.5Al0.5Ti1.5(PO 4)3 solid electrolyte via a sol-gel route using various Al sources. Ceram Int 39:4645–4649. https://doi.org/10.1016/j.ceramint.2012.10.206
Liu J, Liu T, Pu Y, Guan M, Tang Z, Ding F, Xu Z, Li Y (2017) Facile synthesis of NASICON-type Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte and its application for enhanced cyclic performance in lithium ion batteries through the introduction of an artificial Li3PO4 SEI layer. RSC Adv 7:46545–46552. https://doi.org/10.1039/c7ra09335g
Tolganbek N, Mentbayeva A, Uzakbaiuly B, Kanamura K, Bakenov Z (2019) Li1+xAlxTi2-x (PO4)3, NASICON-type solid electrolyte fabrication with different methods. Materials Today: Proceedings. Elsevier Ltd, 97–100 https://doi.org/10.1016/j.matpr.2019.12.279
Breuer S, Prutsch D, Ma Q, Epp V, Preishuber-Pflügl F, Tietz F, Wilkening M (2015) Separating bulk from grain boundary Li ion conductivity in the sol-gel prepared solid electrolyte Li1.5Al0.5Ti1.5(PO4)3. J Mater Chem A Mater 3:21343–21350. https://doi.org/10.1039/c5ta06379e
Xingang L, Jiang T, Fu J, Yuan R, Wen H, Zhang C (2017) Facile synthesis of nanosized lithium-ion-conducting solid electrolyte Li1.4Al0.4Ti1.6(PO4)3 and its mechanical nanocomposites with LiMn2O4 for enhanced cyclic performance in lithium ion batteries. ACS Appl Mater Interfaces 9:11696–11703. https://doi.org/10.1021/acsami.6b16233
Xu X, Wen Z, Yang X, Zhang J, Gu Z (2006) High lithium ion conductivity glass-ceramics in Li2O-Al2O3-TiO2-P2O5 from nanoscaled glassy powders by mechanical milling. Solid State Ion 177:2611–2615. https://doi.org/10.1016/j.ssi.2006.04.010
Jie Fu (1997) Superionic conductivity of glass-ceramics in the system Li 2O- Al 2O 3-TiO 2-P 2O 5. Solid State Ionics 96:195–200. https://doi.org/10.1016/S0167-2738(97)00018-0
Hartmann P, Leichtweiss T, Busche MR, Schneider M, Reich M, Sann J, Adelhelm P, Janek J (2013) Degradation of NASICON-type materials in contact with lithium metal: formation of mixed conducting interphases (MCI) on solid electrolytes. J Phys Chem C 117:21064–21074. https://doi.org/10.1021/jp4051275
Funding
This research was supported by the research grants №AP09260012 “The development of red phosphorus-based composite anodes for the next generation lithium-ion and sodium-ion batteries” and №AP08052231 “Development of solid electrolytes with high ionic conductivity for next generation Li-ion batteries” from the Ministry of Education and Science of the Republic of Kazakhstan for 2020–2022.
Author information
Authors and Affiliations
Contributions
Yelnury Baltash: conceptualization; experiments; methodology, overall; investigation; writing—original draft preparation; visualization. Aiym Mashekova: experiments; writing—“Experimental” and “Results and discussion” sections; writing, revision and editing. Mukagali Yegamkulov: experiments, editing. Zhumabay Bakenov: resources. Ivan Trussov: conceptualization; methodology, overall; resources; validation; investigation; data curation; writing-revision and editing. Aliya Mukanova: conceptualization; supervision; resources; funding acquisition; analysis; writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baltash, Y., Mashekova, A., Yegamkulov, M. et al. Silicon doping of LATP via molten flux method. Ionics 29, 2647–2655 (2023). https://doi.org/10.1007/s11581-023-05011-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05011-0