Abstract
Purpose
To evaluate and compare the diagnostic performance of revised contrast-enhanced ultrasound (CEUS) Liver Imaging Reporting and Data System version by combining LR-M category and serum alpha-fetoprotein (AFP) under different cut-off values.
Material and methods
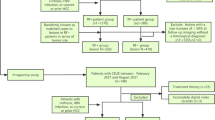
This retrospective study enrolled 152 high-risk patients with 152 histology-proven nodules. For revised LI-RADS, nodules in LR-M with different elevated AFP thresholds have been reclassified as the LR-5 category. The diagnostic performances of original and revised CEUS LI-RADS were evaluated and compared.
Results
To compare with the original version, the sensitivity of revised LR-5 (adjusted with AFP value > 200 ng/ml or 400 ng/ml) for the diagnosis of hepatocellular carcinoma (HCC) improved from 52.5 to 69.2% or 65.0%, respectively (both p < 0.001) without compromising specificity (87.5% vs. 71.9% or 78.1%, respectively, both p > 0.05). For the diagnosis of non-HCC malignancy, the specificity of the LR-M after reclassification was improved (69.6% vs. 84.4% or 80.7%, respectively, both p < 0.001) with a non-significant sensitivity reduction (100.0 vs. 70.6% or 82.4%, respectively, both p > 0.05). After modification, the sensitivity of LR-5 also increased to 69.1% or 64.9% (both p < 0.001), while the specificity and PPV did not change (both p > 0.05) for larger nodules (> 20 mm).
Conclusion
The diagnostic performance of CEUS LI-RADS can be further improved by reclassifying LR-M nodules with elevated AFP thresholds to LR-5.




Similar content being viewed by others
Abbreviations
- ACR:
-
American college of radiology
- AFP:
-
Alpha-fetoprotein
- AUC:
-
Area under the ROC curve
- CEUS:
-
Contrast-enhanced ultrasound
- CHC:
-
Combined hepatocellular cholangiocarcinoma
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- ICC:
-
Intrahepatic cholangiocarcinoma
- LI-RADS:
-
Liver imaging reporting and data system
- MLC:
-
Metastasis liver carcinoma
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- US:
-
Ultrasound
References
McGlynn KA, Petrick JL, London WT (2015) Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 19(2):223–238. https://doi.org/10.1016/j.cld.2015.01.001
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR (2019) A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16(10):589–604. https://doi.org/10.1038/s41575-019-0186-y
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60(6):1268–1289. https://doi.org/10.1016/j.jhep.2014.01.021
Leoni S, Sansone V, Lorenzo S, Ielasi L, Tovoli F, Renzulli M, Golfieri R, Spinelli D, Piscaglia F (2020) Treatment of combined hepatocellular and cholangiocarcinoma. Cancers (Basel). https://doi.org/10.3390/cancers12040794
Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen EL, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX (2013) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM. FLAUS and ICUS Ultraschall Med 34(1):11–29. https://doi.org/10.1055/s-0032-1325499
Schellhaas B, Bernatik T, Dirks K, Jesper D, Mauch M, Potthoff A, Zimmermann P, Strobel D (2021) Contrast-enhanced ultrasound patterns for the non-invasive diagnosis of hepatocellular carcinoma: a prospective multicenter study in histologically proven liver lesions in a real-life setting demonstrating the benefit of extended late phase observation. Ultrasound Med Biol 47(11):3170–3180. https://doi.org/10.1016/j.ultrasmedbio.2021.07.010
Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J (2018) Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer 7(3):235–260. https://doi.org/10.1159/000488035
Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T, Yamakado K, Liver Cancer Study Group of J (2014) JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 3(3–4):458–468. https://doi.org/10.1159/000343875
Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, García-Criado Á, Bianchi L, Belmonte E, Caparroz C, Barrufet M, Bruix J, Brú C (2018) Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol 101:72–81. https://doi.org/10.1016/j.ejrad.2018.01.025
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67(1):358–380. https://doi.org/10.1002/hep.29086
ACR American College of Radiology (2017) Liver Imaging Reporting and Data System. American College of Radiology. https://www.acr.org/Quality-Safety/Resources/LIRADS. Accessed 12 Sep 2017
Bartolotta TV, Terranova MC, Gagliardo C, Taibbi A (2020) CEUS LI-RADS: a pictorial review. Insights Imaging 11(1):9. https://doi.org/10.1186/s13244-019-0819-2
Huang JY, Li JW, Lu Q, Luo Y, Lin L, Shi YJ, Li T, Liu JB, Lyshchik A (2020) Diagnostic accuracy of CEUS LI-RADS for the characterization of liver nodules 20 mm or smaller in patients at risk for hepatocellular carcinoma. Radiology 294(2):329–339. https://doi.org/10.1148/radiol.2019191086
Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, Riccardi L, De Bonis L, Sangiovanni A, Leoni S, Zocco MA, Rossi S, Alessi N, Wilson SR, Piscaglia F, collaborators CL-RIsg (2018) Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol 68(3):485–492. https://doi.org/10.1016/j.jhep.2017.11.007
Zhou H, Zhang C, Du L, Jiang J, Zhao Q, Sun J, Li Q, Wan M, Wang X, Hou X, Wen Q, Liu Y, Zhou X, Huang P (2022) Contrast-enhanced ultrasound liver imaging reporting and data system in diagnosing hepatocellular carcinoma: diagnostic performance and interobserver agreement. Ultraschall Med 43(1):64–71. https://doi.org/10.1055/a-1168-6321
Hanif H, Ali MJ, Susheela AT, Khan IW, Luna-Cuadros MA, Khan MM, Lau DT (2022) Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol 28(2):216–229. https://doi.org/10.3748/wjg.v28.i2.216
Johnson P, Zhou Q, Dao DY, Lo YMD (2022) Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/s41575-022-00620-y
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11(4):317–370. https://doi.org/10.1007/s12072-017-9799-9
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Yang J, Zhang YH, Li JW, Shi YY, Huang JY, Luo Y, Liu JB, Lu Q (2020) Contrast-enhanced ultrasound in association with serum biomarkers for differentiating combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J Gastroenterol 26(46):7325–7337. https://doi.org/10.3748/wjg.v26.i46.7325
Zhang HC, Zhu T, Hu RF, Wu L (2020) Contrast-enhanced ultrasound imaging features and clinical characteristics of combined hepatocellular cholangiocarcinoma: comparison with hepatocellular carcinoma and cholangiocarcinoma. Ultrasonography 39(4):356–366. https://doi.org/10.14366/usg.19093
Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB (2022) The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC). A phase 3 Biomarker study in the United States. Clin Gastroenterol Hepatol. https://doi.org/10.1016/j.cgh.2022.01.047
Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD (2000) Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol 95(6):1535–1538. https://doi.org/10.1111/j.1572-0241.2000.02091.x
Chan SL, Mo F, Johnson PJ, Siu DY, Chan MH, Lau WY, Lai PB, Lam CW, Yeo W, Yu SC (2014) Performance of serum alpha-fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB (Oxford) 16(4):366–372. https://doi.org/10.1111/hpb.12146
Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M (2001) Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 34(4):570–575. https://doi.org/10.1016/s0168-8278(00)00053-2
Schellhaas B, Wildner D, Pfeifer L, Goertz RS, Hagel A, Neurath MF, Strobel D (2016) LI-RADS-CEUS - proposal for a contrast-enhanced ultrasound algorithm for the diagnosis of hepatocellular carcinoma in high-risk populations. Ultraschall Med 37(6):627–634. https://doi.org/10.1055/s-0042-112221
Zeng D, Xu M, Liang JY, Cheng MQ, Huang H, Pan JM, Huang Y, Tong WJ, Xie XY, Lu MD, Kuang M, Chen LD, Hu HT, Wang W (2022) Using new criteria to improve the differentiation between HCC and non-HCC malignancies: clinical practice and discussion in CEUS LI-RADS 2017. Radiol Med 127(1):1–10. https://doi.org/10.1007/s11547-021-01417-w
Zheng W, Li Q, Zou XB, Wang JW, Han F, Li F, Huang LS, Li AH, Zhou JH (2020) Evaluation of contrast-enhanced US LI-RADS version 2017: application on 2020 liver nodules in patients with hepatitis B infection. Radiology 294(2):299–307. https://doi.org/10.1148/radiol.2019190878
Li W, Li L, Zhuang BW, Ruan SM, Hu HT, Huang Y, Lin MX, Xie XY, Kuang M, Lu MD, Chen LD, Wang W (2021) Inter-reader agreement of CEUS LI-RADS among radiologists with different levels of experience. Eur Radiol 31(9):6758–6767. https://doi.org/10.1007/s00330-021-07777-1
Kleiner DE (2018) Hepatocellular carcinoma: liver biopsy in the balance. Hepatology 68(1):13–15. https://doi.org/10.1002/hep.29831
Ding J, Qin Z, Zhou Y, Zhou H, Zhang Q, Wang Y, Jing X, Wang F (2021) Impact of revision of the LR-M criteria on the diagnostic performance of contrast-enhanced ultrasound LI-RADS. Ultrasound Med Biol 47(12):3403–3410. https://doi.org/10.1016/j.ultrasmedbio.2021.08.007
Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX (2019) Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int 39(12):2214–2229. https://doi.org/10.1111/liv.14223
Wen N, Cai Y, Li F, Ye H, Tang W, Song P, Cheng N (2022) The clinical management of hepatocellular carcinoma worldwide: a concise review and comparison of current guidelines: 2022 update. Biosci Trends 16(1):20–30. https://doi.org/10.5582/bst.2022.01061
Zhou Y, Yin S, Zhao L, Zhang X, Li M, Ding J, Yan K, Jing X (2022) CEUS and CT/MRI LI-RADS in association with serum biomarkers for differentiation of combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma. Front Oncol 12:897090. https://doi.org/10.3389/fonc.2022.897090
Li CQ, Huang H, Ruan SM, Hu HT, Xian MF, Xie XY, Lu MD, Kuang M, Wang Y, Chen LD (2022) An assessment of liver lesions using a combination of CEUS LI-RADS and AFP. Abdom Radiol (NY). https://doi.org/10.1007/s00261-022-03428-1
Funding
This work was supported by National Natural Science Foundation of China for Youth Scholars (Grant No. 82202241); Heilongjiang Postdoctoral Science Foundation (Grant No. LBH-Z21022); Innovative Research Project of Harbin Medical University (Grant No. 31041210025).
Author information
Authors and Affiliations
Contributions
Conceptualization: JW; Data curation: WG, RM; Formal analysis: HW, RM; Funding acquisition: HZ; Investigation: JW, CW; Methodology: MW, WX; Project administration: HZ, XZ; Resources: JW, HW; Software: ZJ, WX; Supervision: HZ, XZ; Validation: MW, CW; Visualization: ZJ; Writing-original draft: WG, HZ; Writing-review and editing: WG, JW, HZ; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee the Second Affiliated Hospital of Harbin Medical University.
Ethcial statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Written informed consent was waived by the Institutional Review Board of the Second Affiliated Hospital of Harbin Medical University.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 3 and 4.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, W., Wu, J., Wei, H. et al. Combining serum AFP and CEUS LI-RADS for better diagnostic performance in Chinese high-risk patients. Radiol med 128, 393–401 (2023). https://doi.org/10.1007/s11547-023-01614-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-023-01614-9