Abstract
Purpose
The primary aim of this prospective study was to evaluate the relationship between three-dimensional dynamic contrast-enhanced magnetic resonance (3D-DCE-MR) imaging parameters and clinicopathological features of rectal cancer and assess their potential as new radiological prognostic predictors.
Materials and methods
Three-dimensional DCE-MR was performed on 26 cases of pathologically proved rectal adenocarcinoma 1 week prior to operation. Data were analysed to calculate transfer constant (Ktrans), leakage space (Ve) and rate constant (Kep) of both tumour and normal rectal wall. Microvessel density (MVD) was evaluated by immunohistochemical staining of surgical specimens. All findings were analysed prospectively and correlated with tumour/node/metastasis (TNM) staging, Dukes staging, histological grading, presence of lymph node metastasis, serosal involvement and MVD.
Results
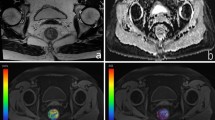
Mean Ktrans, Ve and Kep for tumours were as follows: Ktrans 7.123±3.850/min, Ve 14.2±3.0%, Kep 49.446±20.404/min, revealing the significant difference between the tumour and normal rectal wall (p=0.001). There was a significant difference for Ktrans not only between patients with and without lymphatic involvement (p=0.000), but also among Dukes staging (p=0.04) and pTNM staging (p=0.03). Kep showed moderate correlation with TNM stages (r=0.479, p=0.02). Ve and MVD revealed no significant correlation with the clinicopathological findings described above (p>0.05).
Conclusion
Owing to the moderate and strong relationship between Ktrans and clinicopathological elements, Ktrans might be the prognostic indicator of rectal cancer. Threedimensional DCE high-resolution MR imaging provides a competing opportunity to assess contrast kinetics.
Riassunto
Obiettivo
L’obiettivo principale dello studio è valutare la correlazione tra i parametri ottenuti mediante studio dinamico post-contrasto in risonanza magnetica tridimensionale (3D-DCE-MRI) te le caratteristiche clinico-patologiche del carcinoma rettale e definire le potenzialità di tali parametri di imaging come nuovi fattori prognostici.
Materiali e metodi
Ventisei pazienti affetti da adenocarcinoma del retto, confermato istologicamente, sono stati sottoposti a 3D-DCE-MRI una settimana prima dell’intervento chirurgico. A livello del tessuto tumorale e della parete rettale indenne sono stati calcolati i seguenti parametri: costante di transito (Ktrans), la percentuale dello spazio di distribuzione extravascolare extracellulare — leakage space — (Ve) te la rate constant (Kep). La densità microvascolare è stata stimata mediante analisi immunoistochimica su sezioni del pezzo operatorio. Le analisi sono state condotte in modo prospettico ed i risultati ottenuti correlati alle seguenti caratteristiche clinico-patologiche: stadio di malattia secondo i sistemi TNM e Dukes, grading istologico, presenza di linfonodi metastatici, coinvolgimento della tunica sierosa e densità microvascolare.
Risultati
Dall’analisi del tessuto tumorale sono stati ottenuti i seguenti valori medi di Ktrans, Ve e Kep: Ktrans 7,123±3,850/min, Ve 14,2±3,0 %, Kep 49,446±20,404/min con differenza statisticamente significativa (p=0,001) rispetto ai valori relativi alla parete rettale indenne. Il valore della costante di transito (Ktrans) è risultato significativamente diverso in caso di presenza o meno di coinvolgimento linfonodale di malattia (p=0,000) e nei diversi stadi sia secondo la classificazione di Dukes (p=0.04) che secondo il pTNM (p=0,03). Moderata correlazione è stata dimostrata tra il parametro Kep e gli stadi TNM (r=0,479, p=0,02). Nessuna correlazione statisticamente significativa è stata stabilita per i parametri Ve e MVD (p>0,05).
Conclusioni
La stretta correlazione dimostrata tra i valori di Ktrans e le caratteristiche clinico-patologiche prese in esame rende tale parametro un utile indice prognostico per l’adenocarcinoma del retto. Lo studio dinamico mediante risonanza magnetica 3D rappresenta un valido strumento diagnostico per la valutazione della vascolarizzazione tumorale.
Similar content being viewed by others
References/Bibliografia
World Health Organization, February vn 2006. http://www.who.int/mediacentre/factsheets/fs 297/en/
National Cancer Institute (2009) http://www.cancer.gov/cancertopics/commoncancers
Xu AG, Yu ZJ, Jiang B et al (2010) Colorectal cancer in Guangdong Province of China: A demographic and anatomic survey. World J Gastroenterol 16(8):960–965; DOI: 10.3748/wjg.v16.i8.960
Smith N, Brown G (2008) Preoperative staging of rectal cancer. Acta Oncologica 47:20–31; DOI: 10.1080/02841860701697720
Folkman J (1971) Tumor angiogenesis: therapeutic implications. New Engl J Med 285:1182–1186
Tuncbilek N, Karakas HM, Altaner S (2004) Dynamic MRI in indirect estimation of MVD, histologic grade, and prognosis in colorectal adenocarcinomas. Abdominal Imaging 29:166–172;DOI: 10.1007/s00261-003-0090-2
Brown G, Daniels IR, Richardson C et al (2005) Techniques and troubleshooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol 78(927):245–251; DOI: 10.1259/bjr/33540239
Brown G, Kirkham A, Williams GT et al (2004) High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. Am J Roentgenol 182:431–439; DOI: 0361-803X/04/1822-431
Nicholls RJ, Galloway DJ, Mason AY et al (1985) Clinical local staging of rectal cancer. Br J Surg 72[Suppl]:S51–S2; DOI: 10.1002/bjs.1800721329
Tytherleigh MG, Ng VV, Pittathankal AA et al (2008) Preoperative staging of rectal cancer by MRI remains an imprecise tool. ANZ J Surg 78:194–198; DOI 10.1111/j.1445-2197.2007.04402
Fiocchi F, Iotti V, Ligabue G et al (2010) Contrast-enhanced MRI and PET-CT in the evaluation of patients with suspected local recurrence of rectal carcinoma. Radiol Med DOI 10.1007/s11547-010-0558-4
N. Faccioli, P. Marzola, F. Boschi et al (2007) Pathological animal models in the experimental evaluation of tumour microvasculature with magnetic resonance imaging. Radiol Med 112:319–328;DOI 10.1007/s11547-007-0144-6
Brasch RC, Li KC, Husband JE et al (2000) In vivo monitoring of tumor angiogenesis with MR imaging. Acad Radiol 7:812–823; DOI: 10.1016/S1076-6332(00)80630-3
Goh V, Padhani AR, Rasheed S (2007) Functional imaging of colorectal cancer angiogenesis. Lancet Oncol 8:245–55; DOI 10.1016/S1470-2045 (07)70075-X
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 7:91–101; DOI 10.1002/jmri.1880070113
Parker GJ, Tofts PS (1999) Pharmacokinetic analysis of neoplasms using contrast-enhanced dynamic magnetic resonance imaging. Top Magn Reson Imaging 10(2):130–142; DOI 10.1097/00002142-199904000-00006
Bloch BN, Lenkinski RE, Rofsky NM (2008) The role of magnetic resonance imaging (MRI) in prostate cancer imaging and staging at 1.5 and 3 Tesla: The Beth Israel Deaconess. Medical Center (BIDMC) approach. Cancer Biomarkers 4:251–262
Collins DJ, Padhani AR (2004) Dynamic Magnetic Resonance Imaging of Tumor Perfusion. IEEE Engineering in Medicine and Biology Magazine 23:65–83; DOI 10.1109/MEMB.2004.1360410
Bian J, Sha L, Yang C, Sun CS (2008) Three-dimensional dynamic contrast-enhanced MR angiography for evaluating recipient vessels in orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int 7(5):476–480
Zhong L, Li L, Yao QY. (2005) Preoperative evaluation of pancreaticobiliary tumor using MR multi-imaging techniques. World J Gastroenterol 11(24):3756–3761
Atkin G., Taylor N. J, Daley F M et al (2006) Dynamic contrast-enhanced magnetic resonance imaging is a poor measure of rectal cancer angiogenesis. Br J Surg 93:992–1000; DOI: 10.1002/bjs.5352
Zhang X M, Yu D, Zhang H L et al (2008) 3D Dynamic Contrast-Enhanced MRI of Rectal Carcinoma at 3T: Correlation With Microvascular Density and Vascular Endothelial Growth Factor. Markers of Tumor Angiogenesis. J Magn Reson Imaging 27:1309–1316; DOI 10.1002/jmri.21378
Chen CN, Hsieh FJ, Cheng YM et al (2004) The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Letters 213:73–82; DOI 10.1016/j.canlet.2004.05.020
Weidner N, Semple JP, Welch WR et al (1991) Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Maclean AB, Reid WM, Rolfe KJ et al (2000) Role of angeogenesis in benign, premalignant vulvar lesions. J Reprod Med 45:609–612
George ML, Dzik-Jurasz AS, Padhani AR et al (2001) Non invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg 88:1628–1636; DOI 10.1046/j.0007-1323.2001.01947.x
Jager G.J, Ruijter E T, van de Kaa CA et al (1997) Dynamic Turbo FLASH subtraction technique for contrast enhanced. MR imaging of the prostate: correlation with histopathologic results. Radiology 203:645–652
Anthony T, George R, Rodriguez-Bigas M et al (1996) Primary signet-ring cell carcinoma of the colon and rectum. Ann Surg Oncol 3:344–348; DOI 10.1007/BF02305663
Kim H, Folks KD, Guo Ll et al (2010) DCE-MRI Detects Early Vascular Response in Breast Tumor Xenografts Following Anti-DR5 Therapy. Mol Imaging Biol DOI: 10.1007/s11307-010-0320-2
Benjaminsen IC, Brurberg KG, Ruud EB et al (2008) Assessment of extravascular extracellular space fraction in human melanoma xenografts by DCE-MRI and kinetic modeling. Magn Reson Imaging 26(2):160–170
Kim D J, Kim J H, Lim J S et al (2010) Restaging of Rectal Cancer with MR Imaging after Concurrent Chemotherapy and Radiation Therapy. Radiographics 30:503–516; DOI: 10.1148/rg.302095046
Vandecaveye V, Keyzer FD, Dymarkowski S (2010) Imaging and targeted agents in gastrointestinal cancers: overview on perfusion-and diffusion-weighted magnetic resonance imaging and angiogenesis inhibitors. Targ Oncol DOI 10.1007/s11523-008-0076-7
Saclarides TJ, Speziale NJ, Drab E et al (1994) Tumor angiogenesis and rectal carcinoma. Dis Colon Rectum 37:921–926; DOI 10.1007/BF02052599
Frank RE, Saclarides TJ, Leurgens S et al (1995) Tumor angiogenesis as predictor of recurrence and survival in patients with node negative colon cancer. Ann Surg 222:695–699; DOI: 10.1097/00000658-199512000-00002
Tanigawa N, Amaya H, Matsumura M et al (1997) Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res 57:1043–1046
Svagzdys S, Lesauskaite V, Pavalkis D et al (2009) Microvessel density as new prognostic marker after radiotherapy inrectal cancer. BMC Cancer 9:95–99; DOI: 10.1186/1471-2407-9-95
Cianchi F, Palomba A, Messerini L et al (2002) Tumor angiogenesis in lymph node negative rectal cancer: correlation with clinicopathological parameters and prognosis. Ann Surg Oncol 9:20–26; DOI 10.1245/aso.2002.9.1.20
Lindmark G, Gerdin B, Sundberg C et al (1996) Prognostic significance of microvascular count in colorectal cancer. J Clin Oncol 14:461–466
Abdalla SA, Behzad F, Bsharah S et al (1999) Prognostic relevance of microvessel density in colorectal tumours. Oncol Rep 6:839–842
Abdalla SA, Behzad F, Bsharah S et al (1999) Prognostic relevance of MVD in colorectal tumors. Oncol Rep 6:839–842
Eberhard A, Kahlert S, Goede V et al (2000) Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res 60:1388–1393
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, W.W., Zhang, H., Ding, B. et al. Rectal cancer: 3D dynamic contrast-enhanced MRI; correlation with microvascular density and clinicopathological features. Radiol med 116, 366–374 (2011). https://doi.org/10.1007/s11547-011-0628-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-011-0628-2