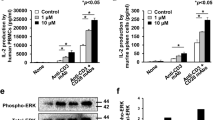
Abstract
Background
Palmitoyl-protein thioesterase-1 (PPT1) is a clinical stage druggable target for inhibiting autophagy in cancer.
Objective
We aimed to determine the cellular and molecular activity of targeting PPT1 using ezurpimtrostat, in combination with an anti-PD-1 antibody.
Methods
In this study we used a transgenic immunocompetent mouse model of hepatocellular carcinoma.
Results
Herein, we revealed that inhibition of PPT1 using ezurpimtrostat decreased the liver tumor burden in a mouse model of hepatocellular carcinoma by inducing the penetration of lymphocytes into tumors when combined with anti-programmed death-1 (PD-1). Inhibition of PPT1 potentiates the effects of anti-PD-1 immunotherapy by increasing the expression of major histocompatibility complex (MHC)-I at the surface of liver cancer cells and modulates immunity through recolonization and activation of cytotoxic CD8+ lymphocytes.
Conclusions
Ezurpimtrostat turns cold tumors into hot tumors and, thus, could improve T cell-mediated immunotherapies in liver cancer.
Graphical Abstract






Similar content being viewed by others
References
Lu JY, Verkruyse LA, Hofmann SL. Lipid thioesters derived from acylated proteins accumulate in infantile neuronal ceroid lipofuscinosis: correction of the defect in lymphoblasts by recombinant palmitoyl-protein thioesterase. Proc Natl Acad Sci USA. 1996;93:10046–50.
Verkruyse LA, Hofmann SL. Lysosomal targeting of palmitoyl-protein thioesterase. J Biol Chem. 1996;271:15831–6.
Bagh MB, Peng S, Chandra G, Zhang Z, Singh SP, Pattabiraman N, et al. Misrouting of v-ATPase subunit V0a1 dysregulates lysosomal acidification in a neurodegenerative lysosomal storage disease model. Nat Commun. 2017;8:14612.
Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303.
Futai M, Sun-Wada G-H, Wada Y, Matsumoto N, Nakanishi-Matsui M. Vacuolar-type ATPase: A proton pump to lysosomal trafficking. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95:261–77.
Yap SQ, Mathavarajah S, Huber RJ. The converging roles of Batten disease proteins in neurodegeneration and cancer. iScience. 2021;24:102337.
Rebecca VW, Nicastri MC, Fennelly C, Chude CI, Barber-Rotenberg JS, Ronghe A, et al. PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. Cancer Discov. 2019;9:220–9.
Rebecca VW, Nicastri MC, McLaughlin N, Fennelly C, McAfee Q, Ronghe A, et al. A unified approach to targeting the lysosome’s degradative and growth signaling roles. Cancer Discov. 2017;7:1266–83.
Brun S, Bestion E, Raymond E, Bassissi F, Jilkova ZM, Mezouar S, et al. GNS561, a clinical-stage PPT1 inhibitor, is efficient against hepatocellular carcinoma via modulation of lysosomal functions. Autophagy. 2022;18:678–94.
Potts MB, McMillan EA, Rosales TI, Kim HS, Ou Y-H, Toombs JE, et al. Mode of action and pharmacogenomic biomarkers for exceptional responders to didemnin B. Nat Chem Biol. 2015;11:401–8.
Sharma G, Ojha R, Noguera-Ortega E, Rebecca VW, Attanasio J, Liu S, et al. PPT1 inhibition enhances the antitumor activity of anti-PD-1 antibody in melanoma. JCI Insight. 2020;5(17):e133225. https://doi.org/10.1172/jci.insight.133225.
Wang H, Yao H, Li C, Shi H, Lan J, Li Z, et al. HIP1R targets PD-L1 to lysosomal degradation to alter T cell–mediated cytotoxicity. Nat Chem Biol. 2019;15:42–50.
Mach L, Stüwe K, Hagen A, Ballaun C, Glössl J. Proteolytic processing and glycosylation of cathepsin B. The role of the primary structure of the latent precursor and of the carbohydrate moiety for cell-type-specific molecular forms of the enzyme. Biochem J. 1992;282:577–82.
Harding JJ, Awada A, Roth G, Decaens T, Merle P, Kotecki N, et al. First-in-human effects of PPT1 inhibition using the oral treatment with GNS561/ezurpimtrostat in patients with primary and secondary liver cancers. Liver Cancer. 2022;11:268–77.
Brun S, Bassissi F, Serdjebi C, Novello M, Tracz J, Autelitano F, et al. GNS561, a new lysosomotropic small molecule, for the treatment of intrahepatic cholangiocarcinoma. Invest New Drugs. 2019;37:1135–45.
Dupuy E, Hainaud P, Villemain A, Bodevin-Phèdre E, Brouland JP, Briand P, et al. Tumoral angiogenesis and tissue factor expression during hepatocellular carcinoma progression in a transgenic mouse model. J Hepatol. 2003;38:793–802.
Bonnin P, Villemain A, Vincent F, Debbabi H, Silvestre JS, Contreres JO, et al. Ultrasonic assessment of hepatic blood flow as a marker of mouse hepatocarcinoma. Ultrasound Med Biol. 2007;33:561–70.
Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–5.
Chen Y-W, Pan H-B, Tseng H-H, Hung Y-T, Huang J-S, Chou C-P. Assessment of blood flow in hepatocellular carcinoma: correlations of computed tomography perfusion imaging and circulating angiogenic factors. Int J Mol Sci. 2013;14:17536–52.
Bian J, Lin J, Long J, Yang X, Yang X, Lu X, et al. T lymphocytes in hepatocellular carcinoma immune microenvironment: insights into human immunology and immunotherapy. Am J Cancer Res. 2020;10:4585–606.
Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–42.
Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–7.
Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–36.
Maimela NR, Liu S, Zhang Y. Fates of CD8+ T cells in tumor microenvironment. Comput Struct Biotechnol J. 2019;17:1–13.
Tau GZ, Cowan SN, Weisburg J, Braunstein NS, Rothman PB. Regulation of IFN-gamma signaling is essential for the cytotoxic activity of CD8+ T cells. J Immunol. 2001;167:5574–82.
Chen D, Xie J, Fiskesund R, Dong W, Liang X, Lv J, et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun. 2018;9:873.
Mezouar S, Mege J. Changing the paradigm of IFN-γ at the interface between innate and adaptive immunity: macrophage-derived IFN-γ. J Leukoc Biol. 2020;108:419–26.
Najafi M, Hashemi Goradel N, Farhood B, Salehi E, Nashtaei MS, Khanlarkhani N, et al. Macrophage polarity in cancer: a review. J Cell Biochem. 2019;120:2756–65.
Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, et al. Kinase regulation of human MHC Class I molecule expression on cancer cells. Cancer Immunol Res. 2016;4:936–47.
Morrison BJ, Steel JC, Morris JC. Reduction of MHC-I expression limits T-lymphocyte-mediated killing of cancer-initiating cells. BMC Cancer. 2018;18:469.
Zeng H, Zhang W, Gong Y, Xie C. Radiotherapy activates autophagy to increase CD8+ T cell infiltration by modulating major histocompatibility complex class-I expression in non-small cell lung cancer. J Int Med Res. 2019;47:3818–30.
Loi M, Müller A, Steinbach K, Niven J, Barreira da Silva R, Paul P, et al. Macroautophagy proteins control MHC Class I levels on dendritic cells and shape anti-viral CD8+ T cell responses. Cell Rep. 2016;15:1076–87.
Cornel AM, Mimpen IL, Nierkens S. MHC Class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers. 2020;12:1760.
Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61.
McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell. 2017;171:1259-1271.e11.
Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med. 2018;10:eaar3342.
Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:eaan2507.
Rasmussen NL, Kournoutis A, Lamark T, Johansen T. NBR1: The archetypal selective autophagy receptor. J Cell Biol. 2022;221: e202208092.
Adolphe F, Ferlicot S, Verkarre V, Posseme K, Couvé S, Garnier P, et al. Germline mutation in the NBR1 gene involved in autophagy detected in a family with renal tumors. Cancer Genet. 2021;258–259:51–6.
Marsh T, Kenific CM, Suresh D, Gonzalez H, Shamir ER, Mei W, et al. Autophagic degradation of NBR1 restricts metastatic outgrowth during mammary tumor progression. Dev Cell. 2020;52:591-604.e6.
Kenific CM, Debnath J. NBR1-dependent selective autophagy is required for efficient cell-matrix adhesion site disassembly. Autophagy. 2016;12:1958–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Genoscience Pharma.
Conflicts of Interest/Competing Interests
E.B., M.R., S.M., C.A., E.R., and P.H. are employees of Genoscience Pharma. E.R. and P.H. are shareholders of Genoscience Pharma. A.T.R. has no conflict of interest that might be relevant to the contents of this manuscript.
Ethics Approval
All experiments were performed following Directive 2010/63/EU of the European Parliament and Council on September 22, 2010. This project was approved by the local ethic committee (Comité d’éthique en experimentation animale Lariboisière-Villemin n°9).
Consent to Participate
Not applicable.
Consent for Publication
All authors have approved the last version of the manuscript and its future publication.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Authors’ Contributions
Conception and design: EB, MR, AT-R, SM. Data analysis: EB, MR, SM. Study supervision: MR, EB, SM, PH. Writing, review of the manuscript: MR, EB, GR, TD, CA, SM, ER, PH.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bestion, E., Rachid, M., Tijeras-Raballand, A. et al. Ezurpimtrostat, A Palmitoyl-Protein Thioesterase-1 Inhibitor, Combined with PD-1 Inhibition Provides CD8+ Lymphocyte Repopulation in Hepatocellular Carcinoma. Targ Oncol 19, 95–106 (2024). https://doi.org/10.1007/s11523-023-01019-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-01019-8