Abstract
Background
Anaplastic lymphoma kinase-tyrosine kinase inhibitors (ALK-TKI; ALKi) have shown potent antitumor activity in metastatic non-small-cell lung cancer (NSCLC) with ALK rearrangement (ALK+); however, their efficacy in neoadjuvant settings has been poorly explored.
Objective
This retrospective study aimed to examine the clinical activity and tumor immune microenvironment (TIME) changes of neoadjuvant ALKi therapy.
Methods
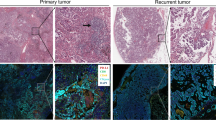
ALK+ NSCLC patients treated with neoadjuvant ALKi at three hospitals in China between February 2018 and January 2023 were assessed. Data on clinical features and radiographic and pathological responses were collected and evaluated. Multiplex immunofluorescence was performed on pretreatment biopsy specimens and surgically resected specimens to investigate the impact of ALKi on TIME.
Results
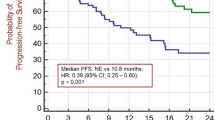
A total of 12 patients with stage IIA–IIIB NSCLC who received neoadjuvant ALKi therapy were analyzed. The objective response rate was 91.7% (11/12) and the major pathological response (MPR) rate was 75.0% (9/12), with 58.3% (7/12) achieving a pathological complete response (pCR). After neoadjuvant ALKi therapy, we observed a significant increase in immune infiltration of CD8+ cells (histochemistry score [H-score]: median 10.51 vs. 24.01, p = 0.028; density: median 128.38 vs. 694.09 cells/mm2, p = 0.028; percentage: median 3.53% vs. 15.92%, p = 0.028) and CD4+ cells (density: median 275.56 vs. 651.82 cells/mm2, p = 0.028; percentage: median 5.98% vs. 10.46%, p = 0.028). Similar results were found for CD4+FOXP3+, CD8+PD1+, CD8+PD1-, CD8+GB+, and CD8+GB- cells. However, macrophages, including CD68+CD163- M1 and CD68+CD163+ M2 macrophages, showed little change after neoadjuvant ALKi therapy.
Conclusion
Neoadjuvant ALKi therapy achieved an encouraging MPR rate of 75% and enhanced immune infiltration, suggesting its safety and feasibility for ALK+ resectable NSCLC. This study advances our understanding of TIME changes by neoadjuvant ALKi therapy and merits further investigation.





Similar content being viewed by others
References
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le Chevalier T, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375(9722):1267–77. https://doi.org/10.1016/s0140-6736(10)60059-1.
NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383(9928):1561–71. https://doi.org/10.1016/s0140-6736(13)62159-5.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. https://doi.org/10.1056/NEJMoa1006448.
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl j Med. 2014;371:2167–77.
Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12(11):1004–12. https://doi.org/10.1016/s1470-2045(11)70232-7.
Zhang C, Shao-Lei Li Q, Nie SD, Shao Y, Yang XN, et al. Neoadjuvant crizotinib in resectable locally advanced non-small cell lung cancer with ALK rearrangement. J Thorac Oncol. 2019;14(4):726–31. https://doi.org/10.1016/j.jtho.2018.10.161.
Zenke Y, Yoh K, Sakakibara-Konishi J, Daga H, Hosomi Y, Nogami N, et al. P1.18–04 neoadjuvant ceritinib for locally advanced non-small cell lung cancer with ALK rearrangement: SAKULA trial. J Thorac Oncol. 2019;14(10 Suppl):S626. https://doi.org/10.1016/j.jtho.2019.08.1320.
Mouillet G, Monnet E, Milleron B, Puyraveau M, Quoix E, David P, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol. 2012;7(5):841–9. https://doi.org/10.1097/JTO.0b013e31824c7d92.
Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–86. https://doi.org/10.1038/s41591-018-0337-7.
Cascone T, William WN Jr, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27(3):504–14. https://doi.org/10.1038/s41591-020-01224-2.
Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–61. https://doi.org/10.1038/s41591-018-0198-0.
Peng H, Wu X, Zhong R, Yu T, Cai X, Liu J, et al. Profiling tumor immune microenvironment of non-small cell lung cancer using multiplex immunofluorescence. Front Immunol. 2021;12:750046. https://doi.org/10.3389/fimmu.2021.750046.
Travis WD, Dacic S, Wistuba I, Sholl L, Adusumilli P, Bubendorf L, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709–40. https://doi.org/10.1016/j.jtho.2020.01.005.
Sun B, Laberiano-Fernández C, Salazar-Alejo R, Zhang J, Solorzano Rendon JL, Lee J, et al. Impact of region-of-interest size on immune profiling using multiplex immunofluorescence tyramide signal amplification for paraffin-embedded tumor tissues. Pathobiology. 2023;90(1):1–12. https://doi.org/10.1159/000523751.
Rizvi NA, Rusch V, Pao W, Chaft JE, Ladanyi M, Miller VA, et al. Molecular characteristics predict clinical outcomes: prospective trial correlating response to the EGFR tyrosine kinase inhibitor gefitinib with the presence of sensitizing mutations in the tyrosine binding domain of the EGFR gene. Clin Cancer Res. 2011;7(10):3500–6. https://doi.org/10.1158/1078-0432.Ccr-10-2102.
Lv C, Fang W, Wu N, Jiao W, Xu S, Ma H, et al. Osimertinib as neoadjuvant therapy in patients with EGFR-mutant resectable stage II-IIIB lung adenocarcinoma (NEOS): a multicenter, single-arm, open-label phase 2b trial. Lung Cancer. 2023;178:151–6. https://doi.org/10.1016/j.lungcan.2023.02.011.
Zhong W, Yang X, Yan H, Zhang X, Su J, Chen Z, et al. Phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 non-small cell lung cancer based on epidermal growth factor receptor mutation status. J Hematol Oncol. 2015;8:54. https://doi.org/10.1186/s13045-015-0151-3.
Zhong WZ, Yan HH, Chen KN, Chen C, Gu CD, Wang J, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer: final overall survival analysis of the EMERGING-CTONG 1103 randomised phase II trial. Signal Transduct Target Ther. 2023;8(1):76. https://doi.org/10.1038/s41392-022-01286-3.
Kilickap S, Onder S, Dizdar O, Erman M, Uner A. Short-time use of crizotinib as neoadjuvant in ALK-positive non-small cell lung carcinoma can be a chance for resectability. Cancer Chemother Pharmacol. 2019;83(6):1195–6. https://doi.org/10.1007/s00280-019-03810-9.
Zhang C, Yan LX, Jiang BY, Wu YL, Zhong WZ. Feasibility and safety of neoadjuvant alectinib in a patient with ALK-positive locally advanced NSCLC. J Thorac Oncol. 2020;15(6):e95–9. https://doi.org/10.1016/j.jtho.2019.12.133.
Zhang C, Wu YL, Zhong WZ. Rapid postoperative relapse in ALK-positive locally advanced NSCLC patient with complete pathological response to neoadjuvant crizotinib. J Thorac Oncol. 2019;14(10):e234–6. https://doi.org/10.1016/j.jtho.2019.05.036.
Bing Z, Jia Z, Wang Y, Xue J, Cao L, Cao Z, et al. Pathological complete response to neoadjuvant ceritinib of a crizotinib-resistant, stage IIIB non-small cell lung cancer with ALK rearrangement: a case report. Thorac Cancer. 2021;12(14):2130–3. https://doi.org/10.1111/1759-7714.14045.
Leonetti A, Minari R, Boni L, Gnetti L, Verzè M, Ventura L, et al. Phase II, open-label, single-arm, multicenter study to assess the activity and safety of alectinib as neoadjuvant treatment in surgically resectable stage III ALK-positive NSCLC: ALNEO trial. Clin Lung Cancer. 2021;22(5):473–7. https://doi.org/10.1016/j.cllc.2021.02.014.
Pyo KH, Lim SM, Park CW, Jo HN, Kim JH, Yun MR, et al. Comprehensive analyses of immunodynamics and immunoreactivity in response to treatment in ALK-positive non-small-cell lung cancer. J Immunother Cancer. 2020;8(2):e000970. https://doi.org/10.1136/jitc-2020-000970.
Maynard A, McCoach CE, Rotow JK, Harris L, Haderk F, Kerr DL, et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell. 2020;182(5):1232-1251.e22. https://doi.org/10.1016/j.cell.2020.07.017.
Fang Y, Wang Y, Zeng D, Zhi S, Shu T, Huang N, et al. Comprehensive analyses reveal TKI-induced remodeling of the tumor immune microenvironment in EGFR/ALK-positive non-small-cell lung cancer. Oncoimmunology. 2021;10(1):1951019. https://doi.org/10.1080/2162402x.2021.1951019.
Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153–67. https://doi.org/10.1038/nri.2017.108.
Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–46. https://doi.org/10.1016/j.immuni.2007.08.014.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by the National Natural Science Foundation of China (82272908); Natural Science Foundation of Chongqing Municipality (CSTB2022NSCQ-MSX1110); The Science Foundation for Outstanding Young People of the Army Medical University (40137-2780); The Science Foundation of Daping Hospital, Army Medical University (2019CXLCB011); Guangdong Association of Clinical Trials (GACT)/Chinese Thoracic Oncology Group (CTONG); Clinical medical technology innovation ability training program (2019CXLCA00); and Clinical Medical Research Talent Training Program of the Army Medical University (2018XLC1015).
Conflict of interest
Nan Zheng, Yimin Zhang, Yue Zeng, Qiang Ma, Ruiguang Zhang, Qian Zhao, Conghua Lu, Jie Tian, ZhiGuo Wang, Huan Tang, Nuo Luo, Hualiang Xiao, Yong He, Fang Wu, and Li Li declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
This study was approved by the Ethics Committee of the Daping Hospital, Army Medical University (no. 2022173).
Consent to participate
Written informed consent was obtained from patients or their legal guardians, before their participation in the study.
Consent for publication
The authors confirm that this manuscript is original and will not be published elsewhere without the written consent of the publisher.
Data availability
All data included in this study are available upon request to the corresponding author.
Code availability
Not applicable.
Authors’ contributions
Conception and design: LL. Development of methodology: FW, YH. Acquisition of data (including acquired and managed patients, and provision of facilities, etc.): NZ, YZ, YZ, QM, RZ, QZ, Conghua Lu, Jie Tian, ZGW, HT, NL, HX. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): NZ, YZ, YZ, CL. Writing, review, and/or revision of the manuscript: NZ, YZ, YZ, QM. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): NZ, YZ, YZ, QM. Study supervision: LL, FW, YH.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, N., Zhang, Y., Zeng, Y. et al. Pathological Response and Tumor Immune Microenvironment Remodeling Upon Neoadjuvant ALK-TKI Treatment in ALK-Rearranged Non-Small Cell Lung Cancer. Targ Oncol 18, 625–636 (2023). https://doi.org/10.1007/s11523-023-00981-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00981-7