Abstract
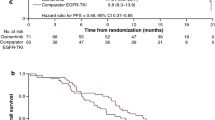
Osimertinib (TAGRISSO®) is an orally administered, third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor that is approved for the adjuvant treatment of adults with completely resected, stage IB–IIIA, EGFR sensitizing mutation (exon 19 deletion or exon 21 [L858R] substitution)-positive non-small cell lung cancer (NSCLC). In the pivotal ADAURA trial in adults with completely resected, early-stage, EGFR mutation-positive (EGFRm+) NSCLC, osimertinib adjuvant therapy significantly prolonged disease-free survival (DFS) compared with placebo in the overall population of patients with stage IB–IIIA disease, as well as in the primary population of patients with stage II–IIIA disease. A DFS benefit of osimertinib was seen irrespective of whether or not patients received prior adjuvant chemotherapy. Overall survival (OS) data were very immature at the time of the analysis of DFS, and more mature OS data are awaited with interest. Osimertinib adjuvant therapy did not adversely affect health-related quality of life and was generally well tolerated, with a manageable safety profile and no new safety signals identified. Based on the available evidence, osimertinib is thus an appropriate targeted option for the adjuvant treatment of adults with completely resected, stage IB–IIIA, EGFRm+ NSCLC.
Plain Language Summary
Almost a third of patients with non-small cell lung cancer (NSCLC) have early-stage disease at diagnosis. Surgical resection is the primary treatment option, with adjuvant chemotherapy also recommended for select individuals with stage IB disease and those with stage II–IIIA disease. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are oral drugs that target and inhibit cancer-driving EGFR sensitizing mutations when present in patients with NSCLC. Osimertinib (TAGRISSO®) is the first EGFR TKI to be approved for adjuvant use in adults with completely resected, stage IB–IIIA, EGFR mutation-positive (EGFRm+) NSCLC. In a trial in the intended patient population, adjuvant osimertinib reduced the risk of disease recurrence or death by ≈ 80% versus placebo, regardless of whether or not patients received adjuvant chemotherapy. The effect of adjuvant osimertinib on overall survival is being evaluated. The safety profile of osimertinib in the early-stage disease setting was consistent with that seen in the advanced disease setting. Based on the available evidence, osimertinib is an appropriate targeted option for the adjuvant treatment of adults with completely resected, stage IB–IIIA, EGFRm+ NSCLC.


Similar content being viewed by others
References
Gabay C, Russo A, Raez LE, et al. Adjuvant therapy in non-small cell lung cancer: is targeted therapy joining the standard of care? Expert Rev Anticancer Ther. 2021;21(11):1229–35.
Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol. 2017;35:2960–74.
Remon J, Soria J-C, Peters S. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32(12):1637–42.
Pignon J-P, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9.
Chevallier M, Borgeaud M, Addeo A, et al. Oncogenic driver mutations in non-small cell lung cancer: past, present and future. World J Clin Oncol. 2021;12(4):217–37.
Abdayem P, Planchard D. Update on molecular pathology and role of liquid biopsy in nonsmall cell lung cancer. Eur Respir Rev. 2021;30:200294.
Passiglia F, Scagliotti GV. The evolving paradigm of precision medicine in lung cancer. Curr Opin Pulm Med. 2021;27(4):249–54.
Pirker R. Adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. Transl Lung Cancer Res. 2014;3(5):305–10.
Kukarni AA, Naqash AR, Puri S, et al. Is it time to implement adjuvant targeted therapy in EGFR-mutant non–small-cell lung cancer? JCO Precis Oncol. 2021;5:408–14.
Chen RL, Sun LL, Cao Y, et al. Adjuvant EGFR-TKIs for patients with resected EGFR-mutant non-small cell lung cancer: a meta-analysis of 1283 patients. Front Oncol. 2021;11:629394.
Lamb YN. Osimertinib: a review in previously untreated, EGFR mutation-positive, advanced NSCLC. Target Oncol. 2021;16:687–95.
Lamb YN, Scott LJ. Osimertinib: a review in T790M-positive advanced non-small cell lung cancer. Target Oncol. 2017;12(4):555–62.
Lee CS, Milone M, Seetharamu N. Osimertinib in EGFR-mutated lung cancer: a review of the existing and emerging clinical data. OncoTargets Ther. 2021;14:4579–97.
Cross DAE, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–61.
AstraZeneca AB. Tagrisso film coated tablets: EU summary of product characteristics. 2020. https://www.ema.europa.eu/. Accessed 18 May 2022.
AstraZeneca Pharmaceuticals LP. Tagrisso - osimertinib tablet, film coated: US prescribing information. 2022. https://www.tagrisso.com/. Accessed 18 May 2022.
Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711–23.
Ballard P, Yates JWT, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–40.
Colclough N, Chen K, Johnström P, et al. Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs. Clin Cancer Res. 2021;27(1):189–201.
Varrone A, Varnäs K, Jucaite A, et al. A PET study in healthy subjects of brain exposure of 11C-labelled osimertinib: a drug intended for treatment of brain metastases in non-small cell lung cancer. J Cereb Blood Flow Metab. 2020;40(4):799–807.
Ekman S, Cselényi Z, Varrone A, et al. A PET and MRI study exploring osimertinib brain exposure and efficacy in EGFRm NSCLC CNS metastases [abstract no. P76.72]. J Thorac Oncol. 2021;16(3 Suppl):S620.
Cho JH, Lim SH, An HJ, et al. Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-LU15-09) clinical trial. J Clin Oncol. 2020;38(5):488–95.
Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study [abstract no. LBA50]. Ann Oncol. 2018;29(Suppl 8):viii740.
Herbst RS, Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB-IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA [abstract no. LBA5 plus oral presentation]. J Clin Oncol. 2020. https://doi.org/10.1200/JCO.2020.38.18_suppl.LBA5.
Majem M, Goldman JW, Thomas J, et al. Health-related quality of life outcomes in patients with resected epidermal growth factor receptor-mutated non-small cell lung cancer who received adjuvant osimertinib in the phase III ADAURA trial. Clin Cancer Res. 2022. https://doi.org/10.1158/1078-0432.CCR-21-3530.
Ewer MS, Tekumalla SH, Walding A, et al. Cardiac safety of osimertinib: a review of data. J Clin Oncol. 2021;39(4):328–37.
AstraZeneca. Tagrisso approved in China in early lung cancer [media release]. 14 Apr 2021. http://www.astrazeneca.com.
Wu Y, John T, Grohe C, et al. Postoperative chemotherapy use and outcomes from ADAURA: osimertinib as adjuvant therapy for resected EGFR-mutated NSCLC. J Thorac Oncol. 2021;17(3):423–33.
Schmid S, Garcia M, Hueniken K, et al. Prevalence, treatment patterns and long-term clinical outcomes of patients with EGFR positive resected stage IB-IIIA NSCLC [abstract no. FP01.03]. In: IASLC 2021 WCLC.
Kuruvilla MS, Syed I, Gwadry-Sridhar F, et al. Real-world outcomes in resected stage IB-IIIA EGFR-mutated NSCLC in Canada: analysis from the POTENT study [poster no. 1152P]. In: ESMO Congress 2021.
Passiglia F, Bertaglia V, Reale ML, et al. Major breakthrough in lung cancer adjuvant treatment: looking beyond the horizon. Cancer Treat Rev. 2021;101:102308.
Melosky B, Cheema P, Juergens RA, et al. The dawn of a new era, adjuvant EGFR inhibition in resected non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:17588359211056306.
Remon J, Hendriks LEL. Osimertinib should be the standard of care for the adjuvant therapy of stage IB to IIIA EGFR-mutant NSCLC. J Thorac Oncol. 2021;16(3):368–70.
West H. ADAURA: a definitive answer to the wrong question. Precis Cancer Med. 2020;3:32.
Gyawali B, West HJ. Lessons from ADAURA on adjuvant cancer drug trials: evidence, ethics, and economics. J Clin Oncol. 2021;39(3):175–7.
Mielgo-Rubio X, Martın M, Remon J, et al. Targeted therapy moves to earlier stages of non-small-cell lung cancer: emerging evidence, controversies and future challenges. Future Oncol. 2021;17(30):4011–25.
Camidge DR. The magic of ADAURA? J Thorac Oncol. 2021;17(3):348–50.
National Comprehensive Cancer Network. NCCN guidelines version 3.2022. Non-small cell lung cancer. 2022. https://www.nccn.org. Accessed 18 May 2022.
Jie GL, Lu HL, Liu SY, et al. The role of adjuvant targeted therapy for postoperative EGFR mutant non-small cell lung cancer: a network meta-analysis [abstract no. 359P]. Ann Oncol. 2020;31(Suppl 6):S1379.
West H. What evidence level is enough for approval and reimbursement of novel cancer drugs? Lessons from lung cancer [abstract no. ES13.04]. J Thorac Oncol. 2021;16(10 Suppl):S834–5.
Uprety D. Osimertinib should not yet be considered the standard of care for EGFR-mutant NSCLC in the adjuvant setting. J Thorac Oncol. 2021;16(3):371–4.
National Institute for Health and Care Excellence. Osimertinib (Tagrisso) for adjuvant treatment of EGFR mutation positive non-small cell lung cancer in adults after complete tumour resection. Technology appraisal guidance [TA761]. 2022. https://www.nice.org.uk. Accessed 18 May 2022.
Scottish Medicines Consortium. Osimertinib 40mg and 80mg film-coated tablets (Tagrisso®): SMC2383. https://www.scottishmedicines.org.uk/. Accessed 18 May 2022.
Lemmon C, Zabor EC, Pennell NA. Modeling the cost-effectiveness of adjuvant osimertinib in resected EGFR-mutant non-small cell lung cancer patients [abstract no. 8527]. J Clin Oncol. 2021;39(Suppl 15):8527.
Acknowledgements
During the peer review process, the manufacturer of osimertinib was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of Interest
James E. Frampton is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: K. Araki, Department of Medical Oncology, Gunma Prefectural Cancer Center, Gunma, Japan; C. Gabay, Ange H. Roffo Cancer Institute, University of Buenos Aires, Buenos Aires, Argentina; C. Grohe, Klinik für Pneumologie - Evangelische Lungenklinik Berlin Buch, Berlin, Germany; F. Passiglia, Department of Oncology, University of Turin, S. Luigi Gonzaga Hospital, Orbassano, Italy; H. West, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Frampton, J.E. Osimertinib: A Review in Completely Resected, Early-Stage, EGFR Mutation-Positive NSCLC. Targ Oncol 17, 369–376 (2022). https://doi.org/10.1007/s11523-022-00883-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00883-0