Abstract
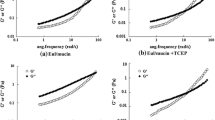
Biopolymer-based nanoparticles, prepared from stearic acid-chitosan derivative, sodium caseinate (NaCas) and oxidized dextran (Odex), have been shown to have high biocompatibility and exceptional gastrointestinal (GI) stability. However, the mucoadhesive properties of such nanoparticles have yet to be analyzed. Therefore, interactions between the nanoparticles and mucin, the major protein of the mucus layer, were evaluated through rheological studies, quartz crystal microbalance with dissipation (QCM-D) analysis, and adsorption testing. There was an increase in the elastic and viscous properties of mucin when complexed with the nanoparticles during the frequency sweep, denoting rheological synergism. The nanoparticles exhibited rapid decreases in frequency and increases in dissipation during the QCM-D analysis, denoting successful adsorption onto the mucin layer. Following adsorption testing, peak adsorption (91 %) was found to be at a ratio of 1:4 at mucin/nanoparticles (weight/weight). Once the role of the nanoparticles’ mucoadhesive properties in GI stability was determined, astaxanthin (ASTX) was utilized as a model nutrient for enhanced bioavailability via encapsulation. The effect of encapsulation on ASTX was determined through measuring particulate properties. Encapsulated ASTX exhibited enhanced antioxidant activity compared to free ASTX in ABTS and DPPH antioxidant assays. This study suggests that the as-prepared biopolymer nanoparticles exhibit mucoadhesive properties that could beneficially interact with the mucus layer of the GI tract. Given their enhanced mucoadhesive properties, the nanoparticles make an excellent candidate for the encapsulation of nutrients with low bioavailability such as the lipophilic ASTX.








Similar content being viewed by others
References
Q. Hu, M. Bae, E. Fleming, J.-Y. Lee, Y. Luo, Food Hydrocoll. 89 (2019). https://doi.org/10.1016/j.foodhyd.2018.10.057
Y. Luo, Colloid Surface B. 196 (2020). https://doi.org/10.1016/j.colsurfb.2020.111309
K. Fuhrmann, G. Fuhrmann, Curr. Opin. Colloid Interface Sci. 31 (2017). https://doi.org/10.1016/j.cocis.2017.07.002
DF Evans, G Pye, R Bramley, AG Clark, TJ Dyson, JD Hardcastle, Gut 29, 1035 (1988). https://doi.org/10.1136/gut.29.8.1035
LM Ensign, R Cone, J Hanes, Adv. Drug Deliv. Rev. 64, 557 (2012). https://doi.org/10.1016/j.addr.2011.12.009
S. Hong, D.W. Choi, H.N. Kim, C.G. Park, W. Lee, H.H. Park, Pharmaceutics 12 (2020). https://doi.org/10.3390/pharmaceutics12070604
M. Bodnar, J.F. Hartmann, J. Borbely, Biomacromolecules 6 (2005). https://doi.org/10.1021/bm0502258
Q. Hu, S. Hu, E. Fleming, J.-Y. Lee, Y. Luo, J. Biol. Macromol. 151 (2020). https://doi.org/10.1016/j.ijbiomac.2020.02.170
Q. Hu, Y. Luo, Carbohydr. Polym. 151 (2016). https://doi.org/10.1016/j.carbpol.2016.05.109
Q Hu, J-Y Lee, Y Luo, Eng. Sci. (2019). https://doi.org/10.30919/es8d507
J. Grießinger, S. Dünnhaupt, B. Cattoz, P. Griffiths, S. Oh, S.B. i Gómez, M. Wilcox, J. Pearson, M. Gumbleton, M. Abdulkarim, I. Pereira de Sousa, Bernkop-Schnürch A, Eur. J. Pharm. Biopharm. 96 (2015). https://doi.org/10.1016/j.ejpb.2015.01.005
L.M. Ensign, C. Schneider, J.S. Suk, R. Cone, J. Hanes, Adv. Mater. 24 (2012). https://doi.org/10.1002/adma.201201800
S.K. Lai, Y.-Y. Wang, D. Wirtz, J. Hanes, Adv. Drug Deliv. Rev. 61 (2009). https://doi.org/10.1016/j.addr.2008.09.012
S.K. Lai, Y.-Y. Wang, J. Hanes, Adv. Drug Deliv. Rev. 61. (2009). https://doi.org/10.1016/j.addr.2008.11.002
J.L. McAuley, S.K. Linden, C.W. Png, R.M. King, H.L. Pennington, S.J. Gendler, T.H. Florin, G.R. Hill, V. Korolik, M.A. McGuckin, J. Clin. Investig. 117 (2007). https://doi.org/10.1172/JCI26705
A. Jachak, S.K. Lai, K. Hida, J.S. Suk, N. Markovic, S. Biswal, P.N. Breysse, J. Hanes, Nanotoxicology 6 (2012). https://doi.org/10.3109/17435390.2011.598244
R. Bansil, B.S. Turner, Curr. Opin. Colloid Interface Sci. 11 (2006). https://doi.org/10.1016/j.cocis.2005.11.001
I. Carlstedt, J.K. Sheehan, Biochem. Soc. Trans. 12 (1984). https://doi.org/10.1042/bst0120615
G.J. Strous, J. Dekker, 27, Crit. Rev. Biochem. Mol. Bio. (1992). https://doi.org/10.3109/10409239209082559
J.D. Snyder, A. Walker, Int. Arch. Allergy Immunol. 82 (1987). https://doi.org/10.1159/000234225
R. Donnelly, R. Shaikh, T. Raj Singh, M. Garland, Ad. Woolfson, J. Pharm. Bioallied Sci. 3 (2011). https://doi.org/10.4103/0975-7406.76478
J. das Neves, M.F. Bahia, M.M. Amiji, B. Sarmento, Expert Opin. Drug Deliv. 8 (2011). https://doi.org/10.1517/17425247.2011.586334
J. SMART, Adv. Drug Deliv. Rev. 57 (2005). https://doi.org/10.1016/j.addr.2005.07.001
R.A. Cone, Adv. Drug Deliv. Rev. 61 (2009). https://doi.org/10.1016/j.addr.2008.09.008
A. Rubinstein, B. Tirosh, Pharm. Res. 11 (1994). https://doi.org/10.1023/A:1018961204325
B. Boddupalli, ZulkarN.K. Mohammed, R. Nath, D. Banji, J. Adv. Pharm. Technol. Res. 1 (2010). https://doi.org/10.4103/0110-5558.76436
C.A. Silva, T.M. Nobre, F.J. Pavinatto, O.N. Oliveira, J. Colloid Interface Sci. 376 (2012). https://doi.org/10.1016/j.jcis.2012.03.027
M.P. Sarparanta, L.M. Bimbo, E.M. Mäkilä, J.J. Salonen, P.H. Laaksonen, A.M.K. Helariutta, M.B. Linder, J.T. Hirvonen, T.J. Laaksonen, H.A. Santos, A.J. Airaksinen, Biomaterials 33 (2012). https://doi.org/10.1016/j.biomaterials.2012.01.029
D.I. Wilson, Eye 32 (2018). https://doi.org/10.1038/eye.2017.267
X. Su, W. Chen, W. Xu, Adv. Mech. Eng. 9 (2017). https://doi.org/10.1177/1687814017699765
F. Madsen, K. Eberth, J.D. Smart, Biomaterials 19 (1998). https://doi.org/10.1016/S0142-9612(98)00037-4
S. Rossi, F. Ferrari, M.C. Bonferoni, C. Caramella, Eur. J. Pharm. Sci. 12 (2001). https://doi.org/10.1016/S0928-0987(00)00194-9
R.G. Riley, J.D. Smart, J. Tsibouklis, P.W. Dettmar, F. Hampson, J.A. Davis, G. Kelly, W.R. Wilber, Int. J. Pharm. 217 (2001). https://doi.org/10.1016/S0378-5173(01)00592-0
N. Anarjan, C.P. Tan, I.A. Nehdi, T.C. Ling, Food Chem. 135 (2012). https://doi.org/10.1016/j.foodchem.2012.05.091
J. Mercke Odeberg, Å Lignell, A. Pettersson, P. Höglund, Eur. J. Pharm. Sci. 19 (2003). https://doi.org/10.1016/S0928-0987(03)00135-0
G. Shu, N. Khalid, Z. Chen, M.A. Neves, C.J. Barrow, M. Nakajima, Food Chem. 255 (2018). https://doi.org/10.1016/j.foodchem.2018.02.062
F. Miao, Y. Geng, D. Lu, J. Zuo, Y. Li, Chin. J. Oceanol. Limnol. 31 (2013). https://doi.org/10.1007/s00343-013-2105-3
G.-L. Jiang, M.-J. Zhu, LWT 106 (2019). https://doi.org/10.1016/j.lwt.2019.02.055
R. Ambati, S.-M. Phang, S. Ravi, R. Aswathanarayana, Mar. Drugs 12 (2014). https://doi.org/10.3390/md12010128
S. Goto, K. Kogure, K. Abe, Y. Kimata, K. Kitahama, E. Yamashita, H. Terada, Biochim. Biophys. Acta Biomembr. 1512 (2001). https://doi.org/10.1016/S0005-2736(01)00326-1
P. Palozza, Nutr. Rev. 56 (2009). https://doi.org/10.1111/j.1753-4887.1998.tb01762.x
K-J Yeum, G Beretta, NI Krinsky, RM Russell, G Aldini, Nutrition 25, 839 (2009). https://doi.org/10.1016/j.nut.2009.01.011
L.A. Pham-Huy, H. He, C. Pham-Huy, Int. J. Biomed. Sci. 4 (2008)
AS Veskoukis, AM Tsatsakis, D Kouretas, Cell. Stress Chaperones 17, 11 (2012). https://doi.org/10.1007/s12192-011-0293-3
F. Zanoni, M. Vakarelova, G. Zoccatelli, Mar. Drugs 17 (2019). https://doi.org/10.3390/md17110627
P. Chandra Bhatt, P. Srivastava, P. Pandey, W. Khan, B.P. Panda, RSC Adv. 6 (2016). https://doi.org/10.1039/C5RA19113K
S. Bharathiraja, P. Manivasagan, Y.-O. Oh, M.S. Moorthy, H. Seo, N.Q. Bui, J. Oh, Int. J. Pharm. 517 (2017). https://doi.org/10.1016/j.ijpharm.2016.12.020
D. Santonocito, G. Raciti, A. Campisi, G. Sposito, A. Panico, E.A. Siciliano, M.G. Sarpietro, E. Damiani, C. Puglia, Nanomaterials 11 (2021). https://doi.org/10.3390/nano11020391
Z. Liu, Y. Jiao, Y. Wang, C. Zhou, Z. Zhang, Adv. Drug Deliv. Rev. 60 (2008). https://doi.org/10.1016/j.addr.2008.09.001
Q. Chen, S. Xu, Q. Liu, J. Masliyah, Z. Xu, Adv. Colloid Interface Sci. 233 (2016). https://doi.org/10.1016/j.cis.2015.10.004
S. Sunoqrot, L. Hasan, A. Alsadi, R. Hamed, O. Tarawneh, Colloids Surf. B 156 (2017). https://doi.org/10.1016/j.colsurfb.2017.05.005
Q. Hu, T. Wang, M. Zhou, J. Xue, Y. Luo, J. Agric. Food Chem. 64 (2016). https://doi.org/10.1021/acs.jafc.6b02255
A. Albanese, P.S. Tang, W.C.W. Chan, Annu. Rev. Biomed. Eng. 14 (2012). https://doi.org/10.1146/annurev-bioeng-071811-150124
Q. Hu, T. Wang, M. Zhou, J. Xue, Y. Luo, Int. J. Biol. Macromol. 92 (2016). https://doi.org/10.1016/j.ijbiomac.2016.07.089
G.E. Yakubov, A. Papagiannopoulos, E. Rat, R.L. Easton, T.A. Waigh, Biomacromol 8 (2007). https://doi.org/10.1021/bm700607w
J. Kočevar-Nared, J. Kristl, J. Šmid-Korbar, Biomaterials 18 (1997). https://doi.org/10.1016/S0142-9612(96)00180-9
M.T. Cook, V.v. Khutoryanskiy, Int. J. Pharm. 495 (2015). https://doi.org/10.1016/j.ijpharm.2015.09.064
R.A.A. Muzzarelli, Carbohydr. Polym. 76 (2009). https://doi.org/10.1016/j.carbpol.2008.11.002
I.-Y. Kim, S.-J. Seo, H.-S. Moon, M.-K. Yoo, I.-Y. Park, B.-C. Kim, C.-S. Cho, Biotechnol. Adv. 26 (2008). https://doi.org/10.1016/j.biotechadv.2007.07.009
M. Sumiyoshi, Y. Kimura, J. Pharm. Pharmacol. 58 (2006). https://doi.org/10.1211/jpp.58.2.0007
C.-M. Lehr, J. Haas, Expert Opin. Biol. Ther. 2 (2002). https://doi.org/10.1517/14712598.2.3.287
X. Zhang, W. Dong, H. Cheng, M. Zhang, Y. Kou, J. Guan, Q. Liu, M. Gao, X. Wang, S. Mao, Asian J. Pharm.l Sci. 14 (2019). https://doi.org/10.1016/j.ajps.2018.09.002
V.M. de Oliveira Cardoso, M.P.D. Gremião, B.S.F. Cury, Int. J. Biol. Macromol. 149 (2020). https://doi.org/10.1016/j.ijbiomac.2020.01.235
JP Pearson, A Allen, DA Hutton, in Glycoprotein Methods and Protocols. ed. by A. By, Corfield (Humana Press, Totowa, NJ, 2000), pp. 99–109
K. Aoki, M. Nakagawa, K. Ichimura, J. Am. Chem. Soc. 122 (2000). https://doi.org/10.1021/ja001790f
A. Anitha, N. Deepa, K.P. Chennazhi, S.V. Nair, H. Tamura, R. Jayakumar, Carbohydr. Polym. 83 (2011). https://doi.org/10.1016/j.carbpol.2010.07.028
C.A. Withers, M.T. Cook, L. Methven, M.A. Gosney, and V. v. Khutoryanskiy, Food Funct. 4 (2013). https://doi.org/10.1039/c3fo60291e
W. Xie, P. Xu, Q. Liu, Bioorganic Med. Chem. Lett. 11 (2001). https://doi.org/10.1016/S0960-894X(01)00285-2
V.A. Alexandrova, G.v. Obukhova, N.S. Domnina, D.A. Topchiev, Macromol. Symp. 144 (1999). https://doi.org/10.1002/masy.19991440138
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fleming, E., Jia, Z., Yang, M. et al. Mucoadhesive Biopolymer Nanoparticles for Encapsulation of Lipophilic Nutrients With Enhanced Bioactivity. Food Biophysics 16, 520–531 (2021). https://doi.org/10.1007/s11483-021-09691-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-021-09691-x