Abstract
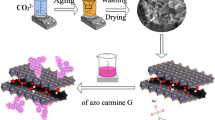
A one-step hydrothermal method was used to synthesize two-dimensional layered Laponite nanosheets (Laponite@diatomite) in situ on the three-dimensional biological template diatomite. The cationic dye removal ability of Laponite@diatomite with different diatomite content was studied. X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), Fourier transformed-infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), and N2 adsorption-desorption technology were used to characterize synthetic materials. The effects of pH, initial concentration, dynamics, and thermodynamics behavior on the adsorption performance were further studied. La@D-0.1 had the highest adsorption efficiency, and the adsorption rate of methylene blue (MB) solution (450 mg L−1) reached 76.7% in the initial 5 min. At the time of adsorption equilibrium (1 h), methylene blue adsorption rate reached 86.4%. The adsorption behavior of La@D-0.1 on MB was characterized by Langumuir isotherm and with better fitting to the quasi-second-order kinetic model curve. By calculating the adsorption process’s thermodynamic parameters, we were able to illustrate that the adsorption process was exothermic. This work provides a novel idea for the synthesis of an economical and ideal, cationic dye nano-adsorbent and provides a potential application for the purification of actual dye wastewater.
Similar content being viewed by others
References
Gao B, Yu H, Wen J, et al. Super-adsorbent poly(acrylic acid)/laponite hydrogel with ultrahigh mechanical property for adsorption of methylene blue. J Environ Chem Eng, 2021, 9: 106346
Liu Y, Wang P, Gojenko B, et al. A review of water pollution arising from agriculture and mining activities in Central Asia: Facts, causes and effects. Environ Pollution, 2021, 291: 118209
Wang W, Wang J, Zhao Y, et al. High-performance two-dimensional montmorillonite supported-poly(acrylamide-co-acrylic acid) hydrogel for dye removal. Environ Pollution, 2020, 257: 113574
Khan M, Lo I M C. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res, 2016, 106: 259–271
Guillossou R, Le Roux J, Mailler R, et al. Influence of dissolved organic matter on the removal of 12 organic micropollutants from wastewater effluent by powdered activated carbon adsorption. Water Res, 2020, 172: 115487
Abdullah N H, Shameli K, Abdullah E C, et al. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos Part B-Eng, 2019, 162: 538–568
Arif M, Liu G, Yousaf B, et al. Synthesis, characteristics and mechanistic insight into the clays and clay minerals-biochar surface interactions for contaminants removal—A review. J Cleaner Production, 2021, 310: 127548
Mukhopadhyay R, Sarkar B, Palansooriya K N, et al. Natural and engineered clays and clay minerals for the removal of poly- and perfluoroalkyl substances from water: State-of-the-art and future perspectives. Adv Colloid Interface Sci, 2021, 297: 102537
Zhang T, Wang W, Zhao Y, et al. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nanocomposites. Chem Eng J, 2021, 420: 127574
Vicente I, Salagre P, Cesteros Y, et al. Fast microwave synthesis of hectorite. Appl Clay Sci, 2009, 43: 103–107
Vicente I, Salagre P, Cesteros Y. Ni nanoparticles supported on microwave-synthesised hectorite for the hydrogenation of styrene oxide. Appl Catal A-General, 2011, 408: 31–37
Bian L, Song M-X, Dong F-Q, et al. DFT and two-dimensional correlation analysis for evaluating the oxygen defect mechanism of low-density 4f (or 5f) elements interacting with Ca-Mt. RSC Adv, 2015, 5: 28601–28610
Yue D, Jing Y, Ma J, et al. Removal of Neutral Red from aqueous solution by using modified hectorite. Desalination, 2011, 267: 9–15
Ma J, Jia Y, Jing Y, et al. Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dyes Pigments, 2012, 93: 1441–1446
Hegyesi N, Vad R T, Pukánszky B. Determination of the specific surface area of layered silicates by methylene blue adsorption: The role of structure, pH and layer charge. Appl Clay Sci, 2017, 146: 50–55
Zhang Z, Li J, Zhao Y, et al. Synthetic Fe-rich nontronite as a novel activator of bisulfite for the efficient removal of tetracycline. J Environ Manage, 2022, 302: 114002
Sriram G, Kigga M, Uthappa U T, et al. Naturally available diatomite and their surface modification for the removal of hazardous dye and metal ions: A review. Adv Colloid Interface Sci, 2020, 282: 102198
Liu L, Fang L, Wu F, et al. Self-supported core-shell heterostructure MnO2/NiCo-LDH composite for flexible high-performance supercapacitor. J Alloys Compd, 2020, 824: 153929
Du Y, Wang L, Wang J, et al. Flower-, wire-, and sheet-like MnO2-deposited diatomites: Highly efficient absorbents for the removal of Cr (VI). J Environ Sci, 2015, 29: 71–81
Li K, Hu Z, Zhao R, et al. A multidimensional rational design of nickel-iron sulfide and carbon nanotubes on diatomite via synergistic modulation strategy for supercapacitors. J Colloid Interface Sci, 2021, 603: 799–809
Okada T, Oguri M, Tajima K, et al. Variation in thickness of a layered silicate on spherical silica particles affected HPLC chiral chromatographic resolution. Appl Clay Sci, 2018, 163: 72–80
Long H, Gu P, Jin G, et al. Preparation of diatomite supported calcium ferrite ternary magnetic material and its adsorption of selenite in aqueous solution. Colloids Surfs A: Physicochem Eng Aspects, 2021, 631: 127727
Peng H H, Chen J, Jiang D Y, et al. Synergistic effect of manganese dioxide and diatomite for fast decolorization and high removal capacity of methyl orange. J Colloid Interface Sci, 2016, 484: 1–9
Ma T, Wu Y, Liu N, et al. Hydrolyzed polyacrylamide modified diatomite waste as a novel adsorbent for organic dye removal: Adsorption performance and mechanism studies. Polyhedron, 2020, 175: 114227
Pawar R R, Lalhmunsiama R R, Gupta P, et al. Porous synthetic hectorite clay-alginate composite beads for effective adsorption of methylene blue dye from aqueous solution. Int J Biol Macromol, 2018, 114: 1315–1324
Gohain M B, Pawar R R, Karki S, et al. Development of thin film nanocomposite membrane incorporated with mesoporous synthetic hectorite and MSH@UiO-66-NH2 nanoparticles for efficient targeted feeds separation, and antibacterial performance. J Membrane Sci, 2020, 609: 118212
Li C, Wang M, Xie B, et al. Enhanced properties of diatomite-based composite phase change materials for thermal energy storage. Renew Energy, 2020, 147: 265–274
Sánchez T, Gebretsadik F B, Salagre P, et al. Evaluation of hectorites, synthesized in different conditions, as soot combustion catalysts after impregnation with copper. Appl Clay Sci, 2013, 77–78: 40–45
Ma J, Jia Y, Jing Y, et al. Synthesis and photocatalytic activity of TiO2-hectorite composites. Appl Clay Sci, 2009, 46: 114–116
Islam M A, Angove M J, Morton D W. Recent innovative research on chromium (VI) adsorption mechanism. Environ Nanotechnol Monitoring Manage, 2019, 12: 100267
Zhu L, Shen D, Luo K H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J Hazard Mater, 2020, 389: 122102
Huang H, Liu Z, Yun J, et al. Preparation of Laponite hydrogel in different shapes for selective dye adsorption and filtration separation. Appl Clay Sci, 2021, 201: 105936
Peng M, Nguyen A V, Wang J, et al. A critical review of the model fitting quality and parameter stability of equilibrium adsorption models. Adv Colloid Interface Sci, 2018, 262: 50–68
Hao L, Gao W, Yan S, et al. Functionalized diatomite/oyster shell powder doped electrospun polyacrylonitrile submicron fiber as a high-efficiency adsorbent for removing methylene blue from aqueous solution: Thermodynamics, kinetics and isotherms. J Mol Liquids, 2020, 298: 112022
Liu D, Yuan W, Yuan P, et al. Physical activation of diatomite-templated carbons and its effect on the adsorption of methylene blue (MB). Appl Surf Sci, 2013, 282: 838–843
Zhao Y, Kang S, Qin L, et al. Self-assembled gels of Fe-chitosan/montmorillonite nanosheets: Dye degradation by the synergistic effect of adsorption and photo-Fenton reaction. Chem Eng J, 2020, 379: 122322
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Grant No. 51908092), the Scientific Research Project of Chongqing Academy of Environmental Sciences (Grant No. 2021-014), the Project Supported Graduate Research and Innovation Foundation of Chongqing, China (Grant No. CYS21001), the Fundamental Research Funds for the Central Universities (Grant Nos. 2020CDJXZ001 and 2021CDJJMRH-005), the Joint Funds of the National Natural Science Foundation of China-Guangdong (Grant No. U1801254), the Chongqing Special Postdoctoral Science Foundation (Grant No. XmT2018043), the Chongqing Research Program of Basic Research and Frontier Technology (Grant No. cstc2017jcyjBX0080), the Natural Science Foundation Project of Chongqing for Post-doctor (Grant Nos. cstc2019jcyjbsh0079 and cstc2019jcyjbshX0085), the Technological Projects of Chongqing Municipal Education Commission (Grant No. KJZDK201800801), the Innovative Research Team of Chongqing (Grant No. CXTDG201602014), and the Innovative Technology of New Materials and Metallurgy (Grant No. 2019CDXYCL0031). The authors also thank the Electron Microscopy Center of Chongqing University for Materials Characterizations.
Supporting Information
The supporting information is available online at https://tech.scichina.com and https://link.springer.com. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
Rights and permissions
About this article
Cite this article
Dai, N., Feng, L., Zhao, L. et al. A high-performance adsorbent of 2D Laponite in-situ coated on 3D diatomite for advanced adsorption of cationic dye. Sci. China Technol. Sci. 65, 2304–2316 (2022). https://doi.org/10.1007/s11431-021-1998-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-021-1998-y