Abstract
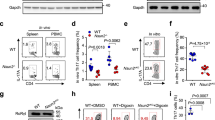
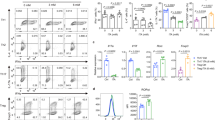
N6-methyladenosine (m6A), the most common and abundant epigenetic RNA modification, governs mRNA metabolism to determine cell differentiation, proliferation and response to stimulation. m6A methyltransferase METTL3 has been reported to control T cell homeostasis and sustain the suppressive function of regulatory T cells (Tregs). However, the role of m6A methyltransferase in other subtypes of T cells remains unknown. T helper cells 17 (Th17) play a pivotal role in host defense and autoimmunity. Here, we found that the loss of METTL3 in T cells caused serious defect of Th17 cell differentiation, and impeded the development of experimental autoimmune encephalomyelitis (EAE). We generated Mettl3f/fIl17aCre mice and observed that METTL3 deficiency in Th17 cells significantly suppressed the development of EAE and displayed less Th17 cell infiltration into central nervous system (CNS). Importantly, we demonstrated that depletion of METTL3 attenuated IL-17A and CCR5 expression by facilitating SOCS3 mRNA stability in Th17 cells, leading to disrupted Th17 cell differentiation and infiltration, and eventually attenuating the process of EAE. Collectively, our results highlight that m6A modification sustains Th17 cell function, which provides new insights into the regulatory network of Th17 cells, and also implies a potential therapeutic target for Th17 cell mediated autoimmune disease.
Similar content being viewed by others
References
Barclay, W., and Shinohara, M.L. (2017). Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Brain Pathol 27, 213–219.
Boriack-Sjodin, P.A., Ribich, S., and Copeland, R.A. (2018). RNA-modifying proteins as anticancer drug targets. Nat Rev Drug Discov 17, 435–453.
Carow, B., and Rottenberg, M.E. (2014). SOCS3, a major regulator of infection and inflammation. Front Immunol 5, 58.
Chen, G., and Shannon, M.F. (2013). Transcription factors and Th17 cell development in experimental autoimmune encephalomyelitis. Crit Rev Immunol 33, 165–182.
Chen, Y., Oh, M.H., Flavell, R., and Li, H.B. (2022). RNA methylation in immune cells. Adv Immunol 155, 39–94.
Cho, J.J., Xu, Z., Parthasarathy, U., Drashansky, T.T., Helm, E.Y., Zuniga, A.N., Lorentsen, K.J., Mansouri, S., Cho, J.Y., Edelmann, M.J., et al. (2019). Hectd3 promotes pathogenic Th17 lineage through Stat3 activation and Malt1 signaling in neuroinflammation. Nat Commun 10, 701.
Ding, C., Xu, H., Yu, Z., Roulis, M., Qu, R., Zhou, J., Oh, J., Crawford, J., Gao, Y., Jackson, R., et al. (2022). RNA m6A demethylase ALKBH5 regulates the development of γδ T cells. Proc Natl Acad Sci USA 119, e2203318119.
Glatigny, S., and Bettelli, E. (2018). Experimental autoimmune encephalomyelitis (EAE) as animal models of multiple sclerosis (MS). Cold Spring Harb Perspect Med 8, a028977.
Gu, S.M., Park, M.H., Yun, H.M., Han, S.B., Oh, K.W., Son, D.J., Yun, J. S., and Hong, J.T. (2016). CCR5 knockout suppresses experimental autoimmune encephalomyelitis in C57BL/6 mice. Oncotarget 7, 15382–15393.
Harris, T.J., Grosso, J.F., Yen, H.R., Xin, H., Kortylewski, M., Albesiano, E., Hipkiss, E.L., Getnet, D., Goldberg, M.V., Maris, C.H., et al. (2007). Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol 179, 4313–4317.
Kaskow, B.J., and Baecher-Allan, C. (2018). Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med 8, a029025.
Kipp, M., Nyamoya, S., Hochstrasser, T., and Amor, S. (2017). Multiple sclerosis animal models: a clinical and histopathological perspective. Brain Pathol 27, 123–137.
Li, H.B., Tong, J., Zhu, S., Batista, P.J., Duffy, E.E., Zhao, J., Bailis, W., Cao, G., Kroehling, L., Chen, Y., et al. (2017). m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548, 338–342.
Liu, J., Harada, B.T., and He, C. (2019). Regulation of gene expression by N6-methyladenosine in cancer. Trends Cell Biol 29, 487–499.
McGinley, A.M., Edwards, S.C., Raverdeau, M., and Mills, K.H.G. (2018). Th17 cells, γδ T cells and their interplay in EAE and multiple sclerosis. J Autoimmun 87, 97–108.
McLoughlin, R.M., Jenkins, B.J., Grail, D., Williams, A.S., Fielding, C.A., Parker, C.R., Ernst, M., Topley, N., and Jones, S.A. (2005). IL-6 trans- signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci USA 102, 9589–9594.
Palmer, D.C., and Restifo, N.P. (2009). Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol 30, 592–602.
Radojcic, V., Pletneva, M.A., Yen, H.R., Ivcevic, S., Panoskaltsis-Mortari, A., Gilliam, A.C., Drake, C.G., Blazar, B.R., and Luznik, L. (2010). STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model. J Immunol 184, 764–774.
Roundtree, I.A., Evans, M.E., Pan, T., and He, C. (2017). Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200.
Shi, H., Wei, J., and He, C. (2019). Where, when, and how: context-dependent functions of RNA Methylation writers, readers, and erasers. Mol Cell 74, 640–650.
Shulman, Z., and Stern-Ginossar, N. (2020). The RNA modification N6-methyladenosine as a novel regulator of the immune system. Nat Immunol 21, 501–512.
Tong, J., Cao, G., Zhang, T., Sefik, E., Amezcua Vesely, M.C., Broughton, J.P., Zhu, S., Li, H., Li, B., Chen, L., et al. (2018). m6A mRNA methylation sustains Treg suppressive functions. Cell Res 28, 253–256.
Wang, Y., Li, L., Li, J., Zhao, B., Huang, G., Li, X., Xie, Z., and Zhou, Z. (2021). The emerging role of m6A modification in regulating the immune system and autoimmune diseases. Front Cell Dev Biol 9, 755691.
Yang, X.O., Panopoulos, A.D., Nurieva, R., Chang, S.H., Wang, D., Watowich, S.S., and Dong, C. (2007). STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 282, 9358–9363.
Yasuda, K., Takeuchi, Y., and Hirota, K. (2019). The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol 41, 283–297.
Yoshimura, A., Naka, T., and Kubo, M. (2007). SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7, 454–465.
Zhou, J., Zhang, X., Hu, J., Qu, R., Yu, Z., Xu, H., Chen, H., Yan, L., Ding, C., Zou, Q., et al. (2021). m6A demethylase ALKBH5 controls CD4+ T cell pathogenicity and promotes autoimmunity. Sci Adv 7, eabg0470.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82230067, 82061148013, 91842105, 81821001), Shanghai Science and Technology Committee (20JC1417400, 201409005500), the National Key Research and Development Program of China (2018YFA0508000), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB29030101), the CAS Project for Young Scientists in Basic Research (YSBR-074), and the Guangdong Provincial Key Laboratory of Tumor Interventional Diagnosis and Treatment (2021B1212040004). We thank all other members of the Hua-Bing Li lab for discussions and comments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no conflict of interest.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Wang, X., Chen, C., Sun, H. et al. m6A mRNA modification potentiates Th17 functions to inflame autoimmunity. Sci. China Life Sci. 66, 2543–2552 (2023). https://doi.org/10.1007/s11427-022-2323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-022-2323-4