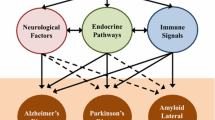
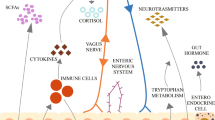
Abstract
The gut microbiota acts as a symbiotic microecosystem that plays an indispensable role in the regulation of a number of metabolic processes in the host by secreting secondary metabolites and impacting the physiology and pathophysiology of numerous organs and tissues through the circulatory system. This relationship, referred to as the “gut-X axis”, is associated with the development and progression of disorders, including obesity, fatty liver and Parkinson’s disease. Given its importance, the gut flora is a vital research area for the understanding and development of the novel therapeutic approaches for multiple disorders. Iron is a common but necessary element required by both mammals and bacteria. As a result, iron metabolism is closely intertwined with the gut microbiota. The host’s iron homeostasis affects the composition of the gut microbiota and the interaction between host and gut microbiota through various mechanisms such as nutrient homeostasis, intestinal peaceability, gut immunity, and oxidative stress. Therefore, understanding the relationship between gut microbes and host iron metabolism is not only of enormous significance to host health but also may offer preventative and therapeutic approaches for a number of disorders that impact both parties. In this review, we delve into the connection between the dysregulation of iron metabolism and dysbiosis of gut microbiota, and how it contributes to the onset and progression of metabolic and chronic diseases.
Similar content being viewed by others
References
Abdeen, A.H., Trist, B.G., and Double, K.L. (2022). Empirical evidence for biometal dysregulation in Parkinson’s disease from a systematic review and Bradford Hill analysis. NPJ Parkinsons Dis 8, 83.
Agirman, G., Yu, K.B., and Hsiao, E.Y. (2021). Signaling inflammation across the gut-brain axis. Science 374, 1087–1092.
Aho, V.T.E., Pereira, P.A.B., Voutilainen, S., Paulin, L., Pekkonen, E., Auvinen, P., and Scheperjans, F. (2019). Gut microbiota in Parkinson’s disease: temporal stability and relations to disease progression. Ebiomedicine 44, 691–707.
Alpizar-Rodriguez, D., Lesker, T.R., Gronow, A., Gilbert, B., Raemy, E., Lamacchia, C., Gabay, C., Finckh, A., and Strowig, T. (2019). Prevotella copri in individuals at risk for rheumatoid arthritis. Ann Rheum Dis 78, 590–593.
Andrews, S.C., Robinson, A.K., and Rodríguez-Quiñones, F. (2003). Bacterial iron homeostasis. FEMS Microbiol Rev 27, 215–237.
Androulidaki, A., Wachsmuth, L., Polykratis, A., and Pasparakis, M. (2018). Differential role of MyD88 and TRIF signaling in myeloid cells in the pathogenesis of autoimmune diabetes. PLoS ONE 13, e0194048.
Aoyama, M., Kotani, J., and Usami, M. (2010). Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26, 653–661.
Asadi Karam, M.R., Habibi, M., and Bouzari, S. (2019). Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol Immunol 108, 56–67.
Aschemeyer, S., Qiao, B., Stefanova, D., Valore, E.V., Sek, A.C., Ruwe, T. A., Vieth, K.R., Jung, G., Casu, C., Rivella, S., et al. (2018). Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 131, 899–910.
Ashraf, A., Jeandriens, J., Parkes, H.G., and So, P.W. (2020). Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: evidence of ferroptosis. Redox Biol 32, 101494.
Ayton, S., Faux, N.G., Bush, A.I., Weiner, M.W., Aisen, P., Petersen, R., Jack, C.R., Jagust, W., Trojanowki, J.Q., Toga, A.W., et al. (2015). Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun 6, 1–9.
Ayton, S., Janelidze, S., Roberts, B., Palmqvist, S., Kalinowski, P., Diouf, I., Belaidi, A.A., Stomrud, E., Bush, A.I., and Hansson, O. (2021). Acute phase markers in CSF reveal inflammatory changes in Alzheimer’s disease that intersect with pathology, APOE ε4, sex and age. Prog Neurobiol 198, 101904.
Ballard, J.W.O., and Towarnicki, S.G. (2020). Mitochondria, the gut microbiome and ROS. Cell Signal 75, 109737.
Bandara, A.B., Drake, J.C., James, C.C., Smyth, J.W., and Brown, D.A. (2021). Complex I protein NDUFS2 is vital for growth, ROS generation, membrane integrity, apoptosis, and mitochondrial energetics. Mitochondrion 58, 160–168.
Barreto, H.C., Abreu, B., and Gordo, I. (2022). Fluctuating selection on bacterial iron regulation in the mammalian gut. Curr Biol 32, 3261–3275.e4.
Behrouzi, B., Weyers, J.J., Qi, X., Barry, J., Rabadia, V., Manca, D., Connelly, J., Spino, M., Wood, J.C., Strauss, B.H., et al. (2020). Action of iron chelator on intramyocardial hemorrhage and cardiac remodeling following acute myocardial infarction. Basic Res Cardiol 115, 1–8.
Belaidi, A.A., Masaldan, S., Southon, A., Kalinowski, P., Acevedo, K., Appukuttan, A.T., Portbury, S., Lei, P., Agarwal, P., Leurgans, S.E., et al. (2022). Apolipoprotein E potently inhibits ferroptosis by blocking ferritinophagy. Mol Psychiatry doi: https://doi.org/10.1038/s41380-022-01568-w.
Bindels, L.B., Beck, R., Schakman, O., Martin, J.C., de Backer, F., Sohet, F. M., Dewulf, E.M., Pachikian, B.D., Neyrinck, A.M., Thissen, J.P., et al., (2012). Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 7, e37971.
Bonaccorsi di Patti, M.C., Cutone, A., Polticelli, F., Rosa, L., Lepanto, M. S., Valenti, P., and Musci, G. (2018). The ferroportin-ceruloplasmin system and the mammalian iron homeostasis machine: regulatory pathways and the role of lactoferrin. Biometals 31, 399–414.
Brissot, P., and Loréal, O. (2016). Iron metabolism and related genetic diseases: A cleared land, keeping mysteries. J Hepatol 64, 505–515.
Britton, L.J., Subramaniam, V.N., and Crawford, D.H. (2016). Iron and non-alcoholic fatty liver disease. World J Gastroenterol 22, 8112.
Brown, R.H., and Al-Chalabi, A. (2017). Amyotrophic lateral sclerosis. N Engl J Med 377, 162–172.
Brune, I., Werner, H., Hüser, A.T., Kalinowski, J., Pühler, A., and Tauch, A. (2006). The DtxR protein acting as dual transcriptional regulator directs a global regulatory network involved in iron metabolism of Corynebacterium glutamicum. BMC Genomics 7, 21.
Byndloss, M.X., Olsan, E.E., Rivera-Chávez, F., Tiffany, C.R., Cevallos, S. A., Lokken, K.L., Torres, T.P., Byndloss, A.J., Faber, F., Gao, Y., et al. (2017). Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575.
Caesar, R., Tremaroli, V., Kovatcheva-Datchary, P., Cani, P.D., and Bäckhed, F. (2015). Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 22, 658–668.
Cai, J., Sun, L., and Gonzalez, F.J. (2022). Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30, 289–300.
Caricilli, A., and Saad, M. (2013). The role of gut microbiota on insulin resistance. Nutrients 5, 829–851.
Carroll, C.S., and Moore, M.M. (2018). Ironing out siderophore biosynthesis: a review of non-ribosomal peptide synthetase (NRPS)-independent siderophore synthetases. Crit Rev Biochem Mol Biol 53, 356–381.
Chávez-Talavera, O., Tailleux, A., Lefebvre, P., and Staels, B. (2017). Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152, 1679–1694.e3.
Che, J., Lv, H., Yang, J., Zhao, B., Zhou, S., Yu, T., and Shang, P. (2021). Iron overload induces apoptosis of osteoblast cells via eliciting ER stress-mediated mitochondrial dysfunction and p-eIF2α/ATF4/CHOP pathway in vitro. Cell Signal 84, 110024.
Chen, F., Yang, D., and Wang, Z. (2021). Associations between iron intake and serum iron with inflammatory bowel disease and chronic diarrheal symptoms in adults: the National Health and Nutrition Examination Survey, 2007–2010. Biol Trace Elem Res 199, 4084–4091.
Chen, J., Li, X., Ge, C., Min, J., and Wang, F. (2022a). The multifaceted role of ferroptosis in liver disease. Cell Death Differ 29, 467–480.
Chen, L., Chen, X.W., Huang, X., Song, B.L., Wang, Y., and Wang, Y. (2019). Regulation of glucose and lipid metabolism in health and disease. Sci China Life Sci 62, 1420–1458.
Chen, Q., Wang, J., Xiang, M., Wang, Y., Zhang, Z., Liang, J., and Xu, J. (2022b). The potential role of ferroptosis in systemic lupus erythematosus. Front Immunol 13, 855622.
Chen, S.J., Chen, C.C., Liao, H.Y., Lin, Y.T., Wu, Y.W., Liou, J.M., Wu, M. S., Kuo, C.H., and Lin, C.H. (2022c). Association of fecal and plasma levels of short-chain fatty acids with gut microbiota and clinical severity in patients with Parkinson disease. Neurology 98, e848–e858.
Cheng, Y., Chen, Y., and Shang, H. (2021). Aberrations of biochemical indicators in amyotrophic lateral sclerosis: a systematic review and meta-analysis. Transl Neurodegener 10, 3.
Chiang, I., Wang, Y., Fujii, S., Muegge, B.D., Lu, Q., Tarr, P.I., and Stappenbeck, T.S. (2021). Biofilm formation and virulence of Shigella flexneri are modulated by pH of gastrointestinal tract. Infect Immun 89, e0038721.
Chopra, V.K., and Anker, S.D. (2020). Anaemia, iron deficiency and heart failure in 2020: facts and numbers. ESC Heart Failure 7, 2007–2011.
Chung The, H., and Le, S.N.H. (2022). Dynamic of the human gut microbiome under infectious diarrhea. Curr Opin Microbiol 66, 79–85.
Corradi, F., Fischetti, I., and De Caterina, R. (2019). Iron and stable ischemic heart disease—lessons from heart failure (in Italian). G Ital Cardiol (Rome) 20, 559–573.
Corrêa-Oliveira, R., Fachi, J.L., Vieira, A., Sato, F.T., and Vinolo, M.A.R. (2016). Regulation of immune cell function by short-chain fatty acids. Clin Trans Immunol 5, e73.
Crépin, R., Goenaga, A.L., Jullienne, B., Bougherara, H., Legay, C., Benihoud, K., Marks, J.D., and Poul, M.A. (2010). Development of human single-chain antibodies to the transferrin receptor that effectively antagonize the growth of leukemias and lymphomas. Cancer Res 70, 5497–5506.
Curran, C.S., Bolig, T., and Torabi-Parizi, P. (2018). Mechanisms and targeted therapies for Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 197, 708–727.
Daily, J.W., and Park, S. (2022). Sarcopenia is a cause and consequence of metabolic dysregulation in aging humans: effects of gut dysbiosis, glucose dysregulation, diet and lifestyle. Cells 11, 338.
Dalile, B., Van Oudenhove, L., Vervliet, B., and Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol 16, 461–478.
Das, N.K., Schwartz, A.J., Barthel, G., Inohara, N., Liu, Q., Sankar, A., Hill, D.R., Ma, X., Lamberg, O., Schnizlein, M.K., et al. (2020). Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab 31, 115–130.e6.
Degen, L., Oesch, S., Casanova, M., Graf, S., Ketterer, S., Drewe, J., and Beglinger, C. (2005). Effect of peptide YY3–36 on food intake in humans. Gastroenterology 129, 1430–1436.
Deng, Q., Yang, S., Sun, L., Dong, K., Li, Y., Wu, S., and Huang, R. (2021). Salmonella effector SpvB aggravates dysregulation of systemic iron metabolism via modulating the hepcidin-ferroportin axis. Gut Microbes 13.
Dewey, K.G., Cohen, R.J., Lönnerdal, B., Domellöf, M., Landa Rivera, L., and Hernell, O. (2002). Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr 132, 3249–3255.
de Sousa, T., Hébraud, M., Dapkevicius, M.L.N.E., Maltez, L., Pereira, J. E., Capita, R., Alonso-Calleja, C., Igrejas, G., and Poeta, P. (2021). Genomic and metabolic characteristics of the pathogenicity in pseudomonas aeruginosa. Int J Mol Sci 22, 12892.
de Vos, W.M., Tilg, H., Van Hul, M., and Cani, P.D. (2022). Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032.
Ding, H., Chen, S., Pan, X., Dai, X., Pan, G., Li, Z., Mai, X., Tian, Y., Zhang, S., Liu, B., et al. (2021). Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J Cachexia Sarcopenia Muscle 12, 746–768.
Diot, C., García-González, A.P., Vieira, A.F., Walker, M., Honeywell, M., Doyle, H., Ponomarova, O., Rivera, Y., Na, H., Zhang, H., et al. (2022). Bacterial diet modulates tamoxifen-induced death via host fatty acid metabolism. Nat Commun 13, 5595.
Donohoe, D.R., Garge, N., Zhang, X., Sun, W., O’Connell, T.M., Bunger, M.K., and Bultman, S.J. (2011). The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13, 517–526.
Duca, F.A., Waise, T.M.Z., Peppler, W.T., and Lam, T.K.T. (2021). The metabolic impact of small intestinal nutrient sensing. Nat Commun 12, 1–2.
El Hout, M., Dos Santos, L., Hamaï, A., and Mehrpour, M. (2018). A promising new approach to cancer therapy: targeting iron metabolism in cancer stem cells. Semin Cancer Biol 53, 125–138.
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 19, 55–71.
Fang, X., Ardehali, H., Min, J., and Wang, F. (2023). The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol 20, 7–23.
Fernández-Real, J.M., Moreno-Navarrete, J.M., and Manco, M. (2019). Iron influences on the gut-brain axis and development of type 2 diabetes. Crit Rev Food Sci Nutr 59, 443–449.
Fielding, R.A., Reeves, A.R., Jasuja, R., Liu, C., Barrett, B.B., and Lustgarten, M.S. (2019). Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp Gerontol 127, 110722.
Foley, K.P., Zlitni, S., Duggan, B.M., Barra, N.G., Anhê, F.F., Cavallari, J. F., Henriksbo, B.D., Chen, C.Y., Huang, M., Lau, T.C., et al. (2020). Gut microbiota impairs insulin clearance in obese mice. Mol Metab 42, 101067.
Franzosa, E.A., Sirota-Madi, A., Avila-Pacheco, J., Fornelos, N., Haiser, H. J., Reinker, S., Vatanen, T., Hall, A.B., Mallick, H., McIver, L.J., et al. (2019). Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol 4, 293–305.
Gajowiak, A., Styś, A., Starzyński, R.R., Staroń, R., and Lipiński, P. (2016). Misregulation of iron homeostasis in amyotrophic lateral sclerosis. Postepy Hig Med Dosw 70, 709–721.
Galaris, D., Barbouti, A., and Pantopoulos, K. (2019). Iron homeostasis and oxidative stress: an intimate relationship. Biochim Biophys Acta 1866, 118535.
Gao, H., Jin, Z., Bandyopadhyay, G., Wang, G., Zhang, D., Rocha, K.C., Liu, X., Zhao, H., Kisseleva, T., Brenner, D.A., et al. (2022d). Aberrant iron distribution via hepatocyte-stellate cell axis drives liver lipogenesis and fibrosis. Cell Metab 34, 1201–1213.e5.
Gao, J., Yang, Z., Zhao, C., Tang, X., Jiang, Q., and Yin, Y. (2023). A comprehensive review on natural phenolic compounds as alternatives to in-feed antibiotics. Sci China Life Sci 66, 1518–1534.
Gao, M., Monian, P., Pan, Q., Zhang, W., Xiang, J., and Jiang, X. (2016). Ferroptosis is an autophagic cell death process. Cell Res 26, 1021–1032.
Gao, M., Yi, J., Zhu, J., Minikes, A.M., Monian, P., Thompson, C.B., and Jiang, X. (2019). Role of mitochondria in ferroptosis. Mol Cell 73, 354–363.e3.
Gao, W., Zhang, T., and Wu, H. (2021). Emerging pathological engagement of ferroptosis in gut diseases. Oxid Med Cell Longev 2021, 4246255.
Garcia-Santos, D., Hamdi, A., Saxova, Z., Fillebeen, C., Pantopoulos, K., Horvathova, M., and Ponka, P. (2018). Inhibition of heme oxygenase ameliorates anemia and reduces iron overload in a β-thalassemia mouse model. Blood 131, 236–246.
Ghafourian, K., Shapiro, J.S., Goodman, L., and Ardehali, H. (2020). Iron and heart failure: diagnosis, therapies, and future directions. JACC Basic Transl Sci 5, 300–313.
Ghanchi, A., James, P.T., and Cerami, C. (2019). Guts, germs, and iron: a systematic review on iron supplementation, iron fortification, and diarrhea in children aged 4–59 months. Curr Devs Nutr 3, nzz005.
Ghouri, Y., Richards, D., Rahimi, E., Krill, J., Jelinek, K.A., and DuPont, A. (2014). Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol 7, 473–487.
Giron, M., Thomas, M., Dardevet, D., Chassard, C., and Savary-Auzeloux, I. (2022). Gut microbes and muscle function: can probiotics make our muscles stronger? J Cachexia Sarcopenia Muscle 13, 1460–1476.
González-Domínguez, Á., Visiedo-García, F.M., Domínguez-Riscart, J., González-Domínguez, R., Mateos, R.M., and Lechuga-Sancho, A.M. (2020). Iron metabolism in obesity and metabolic syndrome. Int J Mol Sci 21, 5529.
Gropper, S.F., Smith, J.K., and Groff, J.L. (2008). Advanced Nutrition and Human Metabolism. Boston: Cengage Learning. 333–337.
Gryzik, M., Asperti, M., Denardo, A., Arosio, P., and Poli, M. (2021). NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochim Biophys Acta 1868, 118913.
Guzy, R.D., Hoyos, B., Robin, E., Chen, H., Liu, L., Mansfield, K.D., Simon, M.C., Hammerling, U., and Schumacker, P.T. (2005). Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1, 401–408.
Haberman, Y., Karns, R., Dexheimer, P.J., Schirmer, M., Somekh, J., Jurickova, I., Braun, T., Novak, E., Bauman, L., Collins, M.H., et al. (2019). Ulcerative colitis mucosal transcriptomes reveal mitochondriopathy and personalized mechanisms underlying disease severity and treatment response. Nat Commun 10, 38.
Hagar, J.A., Powell, D.A., Aachoui, Y., Ernst, R.K., and Miao, E.A. (2014). Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253.
Haifer, C., Paramsothy, S., Kaakoush, N.O., Saikal, A., Ghaly, S., Yang, T., Luu, L.D.W., Borody, T.J., and Leong, R.W. (2022). Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol 7, 141–151.
Halon-Golabek, M., Borkowska, A., Kaczor, J.J., Ziolkowski, W., Flis, D. J., Knap, N., Kasperuk, K., and Antosiewicz, J. (2018). hmSOD1 gene mutation-induced disturbance in iron metabolism is mediated by impairment of Akt signalling pathway. J Cachexia Sarcopenia Muscle 9, 557–569.
Harach, T., Marungruang, N., Duthilleul, N., Cheatham, V., Mc Coy, K.D., Frisoni, G., Neher, J.J., Fåk, F., Jucker, M., Lasser, T., et al. (2017). Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 7, 41802.
Haschka, D., Hoffmann, A., and Weiss, G. (2021). Iron in immune cell function and host defense. Semin Cell Dev Biol 115, 27–36.
He, J., Zhang, P., Shen, L., Niu, L., Tan, Y., Chen, L., Zhao, Y., Bai, L., Hao, X., Li, X., et al. (2020). Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci 21, 6356.
He, L., Li, F., Yin, X., Bohman, P., Kim, S., McClain, C.J., Feng, W., and Zhang, X. (2019). Profiling of polar metabolites in mouse feces using four analytical platforms to study the effects of cathelicidin-related antimicrobial peptide in alcoholic liver disease. J Proteome Res 18, 2875–2884.
Higashida, K., Inoue, S., and Nakai, N. (2020). Iron deficiency attenuates protein synthesis stimulated by branched-chain amino acids and insulin in myotubes. Biochem Biophys Res Commun 531, 112–117.
Hoes, M.F., Grote Beverborg, N., Kijlstra, J.D., Kuipers, J., Swinkels, D. W., Giepmans, B.N.G., Rodenburg, R.J., van Veldhuisen, D.J., de Boer, R.A., and van der Meer, P. (2018). Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 20, 910–919.
Holvoet, T., Joossens, M., Vázquez-Castellanos, J.F., Christiaens, E., Heyerick, L., Boelens, J., Verhasselt, B., van Vlierberghe, H., De Vos, M., Raes, J., et al. (2021). Fecal microbiota transplantation reduces symptoms in some patients with irritable bowel syndrome with predominant abdominal bloating: short- and long-term results from a placebo-controlled randomized trial. Gastroenterology 160, 145–157.e8.
Hood, M.I., and Skaar, E.P. (2012). Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10, 525–537.
Hu, H., Shao, W., Liu, Q., Liu, N., Wang, Q., Xu, J., Zhang, X., Weng, Z., Lu, Q., Jiao, L., et al. (2022). Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat Commun 13, 252.
Huang, C., Yi, P., Zhu, M., Zhou, W., Zhang, B., Yi, X., Long, H., Zhang, G., Wu, H., Tsokos, G.C., et al. (2022). Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: An EXPLORER trial. J Autoimmun 130, 102844.
Ikeda, Y., Imao, M., Satoh, A., Watanabe, H., Hamano, H., Horinouchi, Y., Izawa-Ishizawa, Y., Kihira, Y., Miyamoto, L., Ishizawa, K., et al. (2016). Iron-induced skeletal muscle atrophy involves an Akt-forkhead box O3-E3 ubiquitin ligase-dependent pathway. J Trace Elem Med Biol 35, 66–76.
Ikeda, Y., Satoh, A., Horinouchi, Y., Hamano, H., Watanabe, H., Imao, M., Imanishi, M., Zamami, Y., Takechi, K., Izawa-Ishizawa, Y., et al. (2019). Iron accumulation causes impaired myogenesis correlated with MAPK signaling pathway inhibition by oxidative stress. FASEB J 33, 9551–9564.
Jia, F., Li, H., Jiao, Q., Li, C., Fu, L., Cui, C., Jiang, H., and Zhang, L. (2022). Deubiquitylase OTUD3 prevents Parkinson’s disease through stabilizing iron regulatory protein 2. Cell Death Dis 13, 418.
Jia, W., Xie, G., and Jia, W. (2018). Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 15, 111–128.
Jia, Z., Wu, A., He, M., Zhang, L., Wang, C., and Chen, A. (2019). Metabolites of stable fly reduce diarrhea in mice by modulating the immune system, antioxidants, and composition of gut microbiota. Microb Pathog 134, 103557.
Jiang, M., Li, F., Liu, Y., Gu, Z., Zhang, L., Lee, J., He, L., Vatsalya, V., Zhang, H., Deng, Z., et al. (2023). Probiotic-derived nanoparticles inhibit ALD through intestinal miR194 suppression and subsequent FXR activation. Hepatology 77, 1164–1180.
Jie, Z., Xia, H., Zhong, S.L., Feng, Q., Li, S., Liang, S., Zhong, H., Liu, Z., Gao, Y., Zhao, H., et al. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 8, 845.
Josenhans, C., Müthing, J., Elling, L., Bartfeld, S., and Schmidt, H. (2020). How bacterial pathogens of the gastrointestinal tract use the mucosal glyco-code to harness mucus and microbiota: new ways to study an ancient bag of tricks. Int J Med Microbiol 310, 151392.
Karlsson, F.H., Fåk, F., Nookaew, I., Tremaroli, V., Fagerberg, B., Petranovic, D., Bäckhed, F., and Nielsen, J. (2012). Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3, 1245.
Katsarou, A., and Pantopoulos, K. (2020). Basics and principles of cellular and systemic iron homeostasis. Mol Aspects Med 75, 100866.
Kautz, L., and Nemeth, E. (2014). Molecular liaisons between erythropoiesis and iron metabolism. Blood 124, 479–482.
Kazemian, N., Mahmoudi, M., Halperin, F., Wu, J.C., and Pakpour, S. (2020). Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome 8, 36.
Kim, C.H. (2018). Immune regulation by microbiome metabolites. Immunology 154, 220–229.
Kim, M.H., Kang, S.G., Park, J.H., Yanagisawa, M., and Kim, C.H. (2013). Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145, 396–406.e10.
Kimura, I., Ichimura, A., Ohue-Kitano, R., and Igarashi, M. (2020). Free fatty acid receptors in health and disease. Physiol Rev 100, 171–210.
Kitamura, N., Yokoyama, Y., Taoka, H., Nagano, U., Hosoda, S., Taworntawat, T., Nakamura, A., Ogawa, Y., Tsubota, K., and Watanabe, M. (2021). Iron supplementation regulates the progression of high fat diet induced obesity and hepatic steatosis via mitochondrial signaling pathways. Sci Rep 11, 10753.
Kordus, S.L., Thomas, A.K., and Lacy, D.B. (2022). Clostridioides difficile toxins: mechanisms of action and antitoxin therapeutics. Nat Rev Microbiol 20, 285–298.
Kortman, G.A.M., Boleij, A., Swinkels, D.W., and Tjalsma, H. (2012). Iron availability increases the pathogenic potential of Salmonella Typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS ONE 7, e29968.
Krajmalnik-Brown, R., Ilhan, Z.E., Kang, D.W., and DiBaise, J.K. (2012). Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract 27, 201–214.
Kramer, J., Özkaya, Ö., and Kümmerli, R. (2020). Bacterial siderophores in community and host interactions. Nat Rev Microbiol 18, 152–163.
Kusminski, C.M., Ghaben, A.L., Morley, T.S., Samms, R.J., Adams, A.C., An, Y., Johnson, J.A., Joffin, N., Onodera, T., Crewe, C., et al. (2020). A novel model of diabetic complications: adipocyte mitochondrial dysfunction triggers massive β-cell hyperplasia. Diabetes 69, 313–330.
Lahiri, S., Kim, H., Garcia-Perez, I., Reza, M.M., Martin, K.A., Kundu, P., Cox, L.M., Selkrig, J., Posma, J.M., Zhang, H., et al. (2019). The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med 11.
Lamichhane, S., Sen, P., Alves, M.A., Ribeiro, H.C., Raunioniemi, P., Hyötyläinen, T., and Orešič, M. (2021). Linking gut microbiome and lipid metabolism: moving beyond associations. Metabolites 11, 55.
Lane, D., Bae, D.H., Merlot, A., Sahni, S., and Richardson, D. (2015). Duodenal cytochrome b (DCYTB) in iron metabolism: an update on function and regulation. Nutrients 7, 2274–2296.
Le, J., Zhang, X., Jia, W., Zhang, Y., Luo, J., Sun, Y., and Ye, J. (2019). Regulation of microbiota-GLP1 axis by sennoside A in diet-induced obese mice. Acta Pharm Sin B 9, 758–768.
Le Poul, E., Loison, C., Struyf, S., Springael, J.Y., Lannoy, V., Decobecq, M.E., Brezillon, S., Dupriez, V., Vassart, G., Van Damme, J., et al. (2003). Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278, 25481–25489.
Li, G., Jin, B., and Fan, Z. (2022a). Mechanisms involved in gut microbiota regulation of skeletal muscle. Oxid Med Cell Longev 2022, 2151191.
Li, J., Pan, X., Pan, G., Song, Z., He, Y., Zhang, S., Ye, X., Yang, X., Xie, E., Wang, X., et al. (2020). Transferrin receptor 1 regulates thermogenic capacity and cell fate in brown/beige adipocytes. Adv Sci 7, 1903366.
Li, Y., Ouyang, Q., Chen, W., Liu, K., Zhang, B., Yao, J., Zhang, S., Ding, J., Cong, M., and Xu, A. (2022b). An iron-dependent form of non-canonical ferroptosis induced by labile iron. Sci China Life Sci 66, 516–527.
Liang, J., Zhang, M., Wang, X., Ren, Y., Yue, T., Wang, Z., and Gao, Z. (2021). Edible fungal polysaccharides, the gut microbiota, and host health. Carbohydr Polyms 273, 118558.
Liu, B., Pan, X., Liu, Z., Han, M., Xu, G., Dai, X., Wang, W., Zhang, H., and Xie, L. (2020a). Fecal microbiota as a noninvasive biomarker to predict the tissue iron accumulation in intestine epithelial cells and liver. FASEB J 34, 3006–3020.
Liu, J.J., Wang, X., Zhang, W., Kulyar, M.F.A., Ullah, K., Han, Z., Qin, J., Bi, C., Wang, Y., and Li, K. (2022). Comparative analysis of gut microbiota in healthy and diarrheic yaks. Microb Cell Fact 21, 111.
Liu, L., Guo, H., Song, A., Huang, J., Zhang, Y., Jin, S., Li, S., Zhang, L., Yang, C., and Yang, P. (2020b). Progranulin inhibits LPS-induced macrophage M1 polarization via NF-κB and MAPK pathways. BMC Immunol 21, 32.
Liu, W.C., Fisher, S.M., Wells, J.J.S., Ricca, C.S., Principe, P.A., Trejo, W. H., Bonner, D.P., Gougoutos, J.Z., Toeplitz, B.K., and Sykes, R.B. (1981). Siderochelin, a new ferrous-ion chelating agent produced by Nocardia. J Antibiot 34, 791–799.
Logroscino, G., Gao, X., Chen, H., Wing, A., and Ascherio, A. (2008). Dietary iron intake and risk of Parkinson’s disease. Am J Epidemiol 168, 1381–1388.
Loiseau, L., Ollagnier-de-Choudens, S., Nachin, L., Fontecave, M., and Barras, F. (2003). Biogenesis of Fe-S cluster by the bacterial suf system. J Biol Chem 278, 38352–38359.
Lynch, S.V., and Pedersen, O. (2016). The human intestinal microbiome in health and disease. N Engl J Med 375, 2369–2379.
Ma, X., Das, N.K., Castillo, C., Gourani, A., Perekatt, A.O., Verzi, M.P., and Shah, Y.M. (2019). SMAD family member 3 (SMAD3) and SMAD4 repress HIF2α-dependent iron-regulatory genes. J Biol Chem 294, 3974–3986.
Maeda, Y., Kurakawa, T., Umemoto, E., Motooka, D., Ito, Y., Gotoh, K., Hirota, K., Matsushita, M., Furuta, Y., Narazaki, M., et al. (2016). Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol 68, 2646–2661.
Maeda, Y., and Takeda, K. (2019). Host-microbiota interactions in rheumatoid arthritis. Exp Mol Med 51, 1–6.
Mahadea, D., Adamczewska, E., Ratajczak, A.E., Rychter, A.M., Zawada, A., Eder, P., Dobrowolska, A., and Krela-Kaźmierczak, I. (2021). Iron deficiency anemia in inflammatory bowel diseases—a narrative review. Nutrients 13, 4008.
Mann, J., and Truswell, S. (1971). Essentials of Human Nutrition, Second Edition. Oxford: Oxford University Press.
Mayneris-Perxachs, J., Moreno-Navarrete, J.M., and Fernández-Real, J.M. (2022). The role of iron in host-microbiota crosstalk and its effects on systemic glucose metabolism. Nat Rev Endocrinol 18, 683–698.
McCarthy, E.K., ni Chaoimh, C., Kenny, L.C., Hourihane, J.O.B., Irvine, A. D., Murray, D.M., and Kiely, M.E. (2018). Iron status, body size, and growth in the first 2 years of life. Matern Child Nutr 14, e12458.
Miethke, M., and Marahiel, M.A. (2007). Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71, 413–451.
Miklossy, J., and McGeer, P.L. (2016). Common mechanisms involved in Alzheimer’s disease and type 2 diabetes: a key role of chronic bacterial infection and inflammation. Aging 8, 575–588.
Mittler, R. (2017). ROS are good. Trends Plant Sci 22, 11–19.
Mohamed, R., Cao, Y., Afroz, R., Xu, S., Ta, H.T., Barras, M., Zheng, W., Little, P.J., and Kamato, D. (2020). ROS directly activates transforming growth factor β type 1 receptor signalling in human vascular smooth muscle cells. Biochim Biophys Acta 1864, 129463.
Morales, M., and Xue, X. (2021). Targeting iron metabolism in cancer therapy. Theranostics 11, 8412–8429.
Morales, N.P., Rodrat, S., Piromkraipak, P., Yamanont, P., Paiboonsukwong, K., and Fucharoen, S. (2022). Iron chelation therapy with deferiprone improves oxidative status and red blood cell quality and reduces redox-active iron in β-thalassemia/hemoglobin E patients. Biomed pharmacother 145, 112381.
Mörkl, S., Butler, M.I., Holl, A., Cryan, J.F., and Dinan, T.G. (2020). Probiotics and the microbiota-gut-brain axis: focus on psychiatry. Curr Nutr Rep 9, 171–182.
Mossad, O., Batut, B., Yilmaz, B., Dokalis, N., Mezö, C., Nent, E., Nabavi, L.S., Mayer, M., Maron, F.J.M., Buescher, J.M., et al. (2022). Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat Neurosci 25, 295–305.
Nakamura, T., Naguro, I., and Ichijo, H. (2019). Ironhomeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta 1863, 1398–1409.
Naqvi, S., Asar, T.O., Kumar, V., Al-Abbasi, F.A., Alhayyani, S., Kamal, M.A., and Anwar, F. (2021). A cross-talk between gut microbiome, salt and hypertension. Biomed Pharmacother 134, 111156.
Nie, Y., Wang, Z., Chai, G., Xiong, Y., Li, B., Zhang, H., Xin, R., Qian, X., Tang, Z., Wu, J., et al. (2019). Dehydrocostus lactone suppresses LPS-induced acute lung injury and macrophage activation through NF-κB signaling pathway mediated by p38 MAPK and Akt. Molecules 24, 1510.
Nikolova, V.L., Smith, M.R.B., Hall, L.J., Cleare, A.J., Stone, J.M., and Young, A.H. (2021). Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry 78, 1343–1354.
Nishiwaki, H., Ito, M., Hamaguchi, T., Maeda, T., Kashihara, K., Tsuboi, Y., Ueyama, J., Yoshida, T., Hanada, H., Takeuchi, I., et al. (2022). Short chain fatty acids-producing and mucin-degrading intestinal bacteria predict the progression of early Parkinson’s disease. NPJ Parkinsons Dis 8, 5.
O’Connor, A., O’Morain, C.A., and Ford, A.C. (2017). Population screening and treatment of Helicobacter pylori infection. Nat Rev Gastroenterol Hepatol 14, 230–240.
Otto, B.R., Sparrius, M., Verweij-van Vught, A.M., and MacLaren, D.M. (1990). Iron-regulated outer membrane protein of Bacteroides fragilis involved in heme uptake. Infect Immun 58, 3954–3958.
Paganini, D., and Zimmermann, M.B. (2017). The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr 106, 1688S–1693S.
Pan, Q., Guo, F., Huang, Y., Li, A., Chen, S., Chen, J., Liu, H., and Pan, Q. (2021). Gut microbiota dysbiosis in systemic lupus erythematosus: novel insights into mechanisms and promising therapeutic strategies. Front Immunol 12, 799788.
Paramsothy, S., Kamm, M.A., Kaakoush, N.O., Walsh, A.J., van den Bogaerde, J., Samuel, D., Leong, R.W.L., Connor, S., Ng, W., Paramsothy, R., et al. (2017). Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389, 1218–1228.
Park, M.W., Cha, H.W., Kim, J., Kim, J.H., Yang, H., Yoon, S., Boonpraman, N., Yi, S.S., Yoo, I.D., and Moon, J.S. (2021). NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol 41, 101947.
Parlet, C.P., Brown, M.M., and Horswill, A.R. (2019). Commensal staphylococci influence Staphylococcus aureus skin colonization and disease. Trends Microbiol 27, 497–507.
Parmanand, B.A., Kellingray, L., Le Gall, G., Basit, A.W., Fairweather-Tait, S., and Narbad, A. (2019). A decrease in iron availability to human gut microbiome reduces the growth of potentially pathogenic gut bacteria; an in vitro colonic fermentation study. J Nutr Biochem 7, 20–27.
Pasricha, S.R., Tye-Din, J., Muckenthaler, M.U., and Swinkels, D.W. (2021). Iron deficiency. Lancet 397, 233–248.
Patterson, G.T., Osorio, E.Y., Peniche, A., Dann, S.M., Cordova, E., Preidis, G.A., Suh, J.H., Ito, I., Saldarriaga, O.A., Loeffelholz, M., et al. (2022). Pathologic inflammation in malnutrition is driven by proinflammatory intestinal microbiota, large intestine barrier dysfunction, and translocation of bacterial lipopolysaccharide. Front Immunol 13, 1–6.
Paun, A., Yau, C., and Danska, J.S. (2017). The influence of the microbiome on type 1 diabetes. J Immunol 198, 590–595.
Picca, A., Ponziani, F.R., Calvani, R., Marini, F., Biancolillo, A., Coelho-Júnior, H.J., Gervasoni, J., Primiano, A., Putignani, L., Del Chierico, F., et al. (2020). Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients 12, 65.
Porter, J.L., and Rawla, P. (2022). Hemochromatosis. Treasure Island (FL): StatPearls Publishing.
Porter, N.T., and Martens, E.C. (2017). The critical roles of polysaccharides in gut microbial ecology and physiology. Annu Rev Microbiol 71, 349–369.
Prado, E.L., and Dewey, K.G. (2014). Nutrition and brain development in early life. Nutr Rev 72, 267–284.
Rahbar Saadat, Y., Hejazian, M., Bastami, M., Hosseinian Khatibi, S.M., Ardalan, M., and Zununi Vahed, S. (2018). The role of microbiota in the pathogenesis of lupus: Dose it impact lupus nephritis? Pharmacol Res 139, 191–198.
Rahman, M.M., Islam, F., -Or-Rashid, M.H., Mamun, A.A., Rahaman, M. S., Islam, M.M., Meem, A.F.K., Sutradhar, P.R., Mitra, S., Mimi, A.A., et al. (2022). The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front Cell Infect Microbiol 12, 903570.
Ramírez-Macías, I., Orenes-Piñero, E., Camelo-Castillo, A., Rivera-Caravaca, J.M., López-García, C., and Marín, F. (2022). Novel insights in the relationship of gut microbiota and coronary artery diseases. Crit Rev Food Sci Nutr 62, 3738–3750.
Ramírez-Pérez, O., Cruz-Ramón, V., Chinchilla-López, P., and Méndez-Sánchez, N. (2017). The role of the gut microbiota in bile acid metabolism. Ann Hepatol 16, S21–S26.
Ran, C., Li, Y., Ma, X., Xie, Y., Xie, M., Zhang, Y., Zhou, W., Yang, Y., Zhang, Z., Zhou, L., et al. (2021). Interactions between commensal bacteria and viral infection: insights for viral disease control in farmed animals. Sci China Life Sci 64, 1437–1448.
Rau, M., Rehman, A., Dittrich, M., Groen, A.K., Hermanns, H.M., Seyfried, F., Beyersdorf, N., Dandekar, T., Rosenstiel, P., and Geier, A. (2018). Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur Gastroenterol J 6, 1496–1507.
Rawat, P.S., Seyed Hameed, A.S., Meng, X., and Liu, W. (2022). Utilization of glycosaminoglycans by the human gut microbiota: participating bacteria and their enzymatic machineries. Gut Microbes 14, 2068367.
Rezasoltani, S., Sharafkhah, M., Asadzadeh Aghdaei, H., Nazemalhosseini Mojarad, E., Dabiri, H., Akhavan Sepahi, A., Modarressi, M.H., Feizabadi, M.M., and Zali, M.R. (2018). Applying simple linear combination, multiple logistic and factor analysis methods for candidate fecal bacteria as novel biomarkers for early detection of adenomatous polyps and colon cancer. J Microbiol Methods 155, 82–88.
Rhoades, N.S., Hendrickson, S.M., Prongay, K., Haertel, A., Gill, L., Edwards, R.A., Garzel, L., Slifka, M.K., and Messaoudi, I. (2021). Growth faltering regardless of chronic diarrhea is associated with mucosal immune dysfunction and microbial dysbiosis in the gut lumen. Mucosal Immunol 14, 1113–1126.
Richard, S., Zavaleta, N., Caulfield, L., Black, R., Witzig, R., and Shankar, A. (2006). Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am J Trop Med Hyg 75, 126–132.
Romanowski, K., Zaborin, A., Fernandez, H., Poroyko, V., Valuckaite, V., Gerdes, S., Liu, D.C., Zaborina, O.Y., and Alverdy, J.C. (2011). Prevention of siderophore-mediated gut-derived sepsis due to P. aeruginosa can be achieved without iron provision by maintaining local phosphate abundance: role of pH. BMC Microbiol 11, 1–4.
Rondelli, C.M., Perfetto, M., Danoff, A., Bergonia, H., Gillis, S., O’Neill, L., Jackson, L., Nicolas, G., Puy, H., West, R., et al. (2021). The ubiquitous mitochondrial protein unfoldase CLPX regulates erythroid heme synthesis by control of iron utilization and heme synthesis enzyme activation and turnover. J Biol Chem 297, 100972.
Rueter, C., and Bielaszewska, M. (2020). Secretion and delivery of intestinal pathogenic Escherichia coli virulence factors via outer membrane vesicles. Front Cell Infect Microbiol 10, 1.
Sayin, S.I., Wahlström, A., Felin, J., Jäntti, S., Marschall, H.U., Bamberg, K., Angelin, B., Hyötyläinen, T., Orešič, M., and Bäckhed, F. (2013). Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17, 225–235.
Schoeler, M., and Caesar, R. (2019). Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord 20, 461–472.
Schwartz, A.J., Das, N.K., Ramakrishnan, S.K., Jain, C., Jurkovic, M.T., Wu, J., Nemeth, E., Lakhal-Littleton, S., Colacino, J.A., and Shah, Y.M. (2019). Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J Clin Invest 129, 336–348.
Serino, M. (2019). SCFAs—the thin microbial metabolic line between good and bad. Nat Rev Endocrinol 15, 318–319.
Seyoum, Y., Baye, K., and Humblot, C. (2021). Iron homeostasis in host and gut bacteria—a complex interrelationship. Gut Microbes 13, 1–9.
Shander, A., Cappellini, M.D., and Goodnough, L.T. (2009). Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang 97, 185–197.
Sharma, S., and Tripathi, P. (2019). Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem 63, 101–108.
Sharon, G., Sampson, T.R., Geschwind, D.H., and Mazmanian, S.K. (2016). The central nervous system and the gut microbiome. Cell 167, 915–932.
Shawki, A., Anthony, S.R., Nose, Y., Engevik, M.A., Niespodzany, E.J., Barrientos, T., Öhrvik, H., Worrell, R.T., Thiele, D.J., and Mackenzie, B. (2015). Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am J Physiol Gastrointest Liver Physiol 309, G635–G647.
Shetty, T., Sishtla, K., Park, B., Repass, M.J., and Corson, T.W. (2020). Heme synthesis inhibition blocks angiogenesis via mitochondrial dysfunction. iScience 23, 101391.
Shu, W., Pang, Z., Xu, C., Lin, J., Li, G., Wu, W., Sun, S., Li, J., Li, X., and Liu, Z. (2019). Anti-TNF-α monoclonal antibody therapy improves anemia through downregulating hepatocyte hepcidin expression in inflammatory bowel disease. Mediators Inflamm 2019, 1–13.
Silveira, M.A.D., Bilodeau, S., Greten, T.F., Wang, X.W., and Trinchieri, G. (2022). The gut-liver axis: host microbiota interactions shape hepatocarcinogenesis. Trends Cancer 8, 583–597.
Simpson, C.A., Diaz-Arteche, C., Eliby, D., Schwartz, O.S., Simmons, J.G., and Cowan, C.S.M. (2021). The gut microbiota in anxiety and depression—a systematic review. Clin Psychol Rev 83, 101943.
Sobhani, I., Tap, J., Roudot-Thoraval, F., Roperch, J.P., Letulle, S., Langella, P., Corthier, G., Van Nhieu, J.T., and, Furet, J.P. (2011). Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE 6, e16393.
Soofi, S., Cousens, S., Iqbal, S.P., Akhund, T., Khan, J., Ahmed, I., Zaidi, A.K., and Bhutta, Z.A. (2013). Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 382, 29–40.
Stiban, J., So, M., and Kaguni, L.S. (2016). Iron-sulfur clusters in mitochondrial metabolism: multifaceted roles of a simple cofactor. Biochem Moscow 81, 1066–1080.
Stockwell, B.R., Friedmann Angeli, J.P., Bayir, H., Bush, A.I., Conrad, M., Dixon, S.J., Fulda, S., Gascón, S., Hatzios, S.K., Kagan, V.E., et al. (2017). Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285.
Tang, J., Zhu, Z., He, H., Liu, Z., Xia, Z., Chen, J., Hu, J., Cao, L., Rang, J., Shuai, L., et al. (2021). Bacterioferritin: a key iron storage modulator that affects strain growth and butenyl-spinosyn biosynthesis in Saccharopolyspora pogona. Microb Cell Fact 20, 157.
Ticinesi, A., Nouvenne, A., Cerundolo, N., Catania, P., Prati, B., Tana, C., and Meschi, T. (2019). Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients 11, 1633.
Tilg, H., Adolph, T.E., Dudek, M., and Knolle, P. (2021). Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab 3, 1596–1607.
Tripathi, A., Debelius, J., Brenner, D.A., Karin, M., Loomba, R., Schnabl, B., and Knight, R. (2018). The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 15, 397–411.
Troxell, B., and Hassan, H.M. (2013). Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front Cell Infect Microbiol 3, 59.
Uchida, Y., Kan, H., Sakurai, K., Horimoto, Y., Hayashi, E., Iida, A., Okamura, N., Oishi, K., and Matsukawa, N. (2022). APOE 4 dose associates with increased brain iron and β-amyloid via blood-brain barrier dysfunction. J Neurol Neurosurg Psychiatry 93, 772–778.
Vela, D. (2020). Keeping heart homeostasis in check through the balance of iron metabolism. Acta Physiol 228, 1–8.
Vogt, N.M., Kerby, R.L., Dill-McFarland, K.A., Harding, S.J., Merluzzi, A. P., Johnson, S.C., Carlsson, C.M., Asthana, S., Zetterberg, H., Blennow, K., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7, 1.
Vötsch, D., Willenborg, M., Baumgärtner, W., Rohde, M., and Valentin-Weigand, P. (2020). Bordetella bronchiseptica promotes adherence, colonization, and cytotoxicity of Streptococcus suis in a porcine precision-cut lung slice model. Virulence 12, 84–95.
Wahlström, A., Sayin, S.I., Marschall, H.U., and Bäckhed, F. (2016). Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24, 41–50.
Wang, H., Shi, H., Rajan, M., Canarie, E.R., Hong, S., Simoneschi, D., Pagano, M., Bush, M.F., Stoll, S., Leibold, E.A., et al. (2020a). FBXL5 regulates IRP2 stability in iron homeostasis via an oxygen-responsive [2Fe2S] cluster. Mol Cell 78, 31–41.e5.
Wang, S.Z., Yu, Y.J., and Adeli, K. (2020b). Role of gut microbiota in neuroendocrine regulation of carbohydrate and lipid metabolism via the microbiota-gut-brain-liver axis. Microorganisms 8, 527.
Wang, Y., Dilidaxi, D., Wu, Y., Sailike, J., Sun, X., and Nabi, X. (2020c). Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed Pharmacother 125, 109914.
Wang, Y., Li, A., Liu, J., Mehmood, K., Wangdui, B., Shi, H., Luo, X., Zhang, H., and Li, J. (2019a). L. pseudomesenteroides and L. johnsonii isolated from yaks in Tibet modulate gut microbiota in mice to ameliorate enteroinvasive Escherichia coli-induced diarrhea. Microb Pathog 132, 1–9.
Wang, Y., Wei, J., Zhang, W., Doherty, M., Zhang, Y., Xie, H., Li, W., Wang, N., Lei, G., and Zeng, C. (2022). Gut dysbiosis in rheumatic diseases: A systematic review and meta-analysis of 92 observational studies. Ebiomedicine 80, 104055.
Wang, Z., Yin, W., Zhu, L., Li, J., Yao, Y., Chen, F., Sun, M., Zhang, J., Shen, N., Song, Y., et al. (2018). Iron drives T helper cell pathogenicity by promoting RNA-binding protein PCBP1-mediated proinflammatory cytokine production. Immunity 49, 80–92.e7.
Wang, Z., Zeng, Y.N., Yang, P., Jin, L.Q., Xiong, W.C., Zhu, M.Z., Zhang, J.Z., He, X., and Zhu, X.H. (2019b). Axonal iron transport in the brain modulates anxiety-related behaviors. Nat Chem Biol 15, 1214–1222.
Weersma, R.K., Zhernakova, A., and Fu, J. (2020). Interaction between drugs and the gut microbiome. Gut 69, 1510–1519.
Weiser, J.N., Ferreira, D.M., and Paton, J.C. (2018). Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol 16, 355–367.
Wen, S., Wang, C., Gong, M., and Zhou, L. (2019). An overview of energy and metabolic regulation. Sci China Life Sci 62, 771–790.
Wilkinson, N., and Pantopoulos, K. (2014). The IRP/IRE system in vivo: insights from mouse models. Front Pharmacol 5, 176.
Wu, H., Xie, S., Miao, J., Li, Y., Wang, Z., Wang, M., and Yu, Q. (2020). Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 11, 997–1014.
Wu, R., Li, S., Hudlikar, R., Wang, L., Shannar, A., Peter, R., Chou, P.J., Kuo, H.C.D., Liu, Z., and Kong, A.N. (2022). Redox signaling, mitochondrial metabolism, epigenetics and redox active phytochemicals. Free Radic Biol Med 179, 328–336.
Xiang, H., Chen, S., Zhang, H., Zhu, X., Wang, D., Liu, H., Wang, J., Yin, T., Liu, L., Kong, M., et al. (2021). Removal of roosters alters the domestic phenotype and microbial and genetic profile of hens. Sci China Life Sci 64, 1964–1976.
Xu, E., Chen, M., Zheng, J., Maimaitiming, Z., Zhong, T., and Chen, H. (2018). Deletion of hephaestin and ceruloplasmin induces a serious systemic iron deficiency and disrupts iron homeostasis. Biochem Biophys Res Commun 503, 1905–1910.
Xu, T., Ding, W., Ji, X., Ao, X., Liu, Y., Yu, W., and Wang, J. (2019). Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med 23, 4900–4912.
Yang, S., and Lian, G. (2020). ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem 467, 1–12.
Yang, X., Ai, P., He, X., Mo, C., Zhang, Y., Xu, S., Lai, Y., Qian, Y., and Xiao, Q. (2022a). Parkinson’s disease is associated with impaired gut-blood barrier for short-chain fatty acids. Movement Disord 37, 1634–1643.
Yang, Y., Nguyen, M., Khetrapal, V., Sonnert, N.D., Martin, A.L., Chen, H., Kriegel, M.A., and Palm, N.W. (2022b). Within-host evolution of a gut pathobiont facilitates liver translocation. Nature 607, 563–570.
Yatera, K., and Mukae, H. (2020). Corynebacterium species as one of the major causative pathogens of bacterial pneumonia. Respir Invest 58, 131–133.
Yilmaz, B., and Li, H. (2018). Gut microbiota and iron: the crucial actors in health and disease. Pharmaceuticals 11, 98.
Yoo, J., Groer, M., Dutra, S., Sarkar, A., and McSkimming, D. (2020). Gut microbiota and immune system interactions. Microorganisms 8, 1587.
Yu, Y., Jiang, L., Wang, H., Shen, Z., Cheng, Q., Zhang, P., Wang, J., Wu, Q., Fang, X., Duan, L., et al. (2020). Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 136, 726–739.
Yue, M., Wei, J., Chen, W., Hong, D., Chen, T., and Fang, X. (2022). Neurotrophic role of the next-generation probiotic strain L. lactis MG1363-pMG36e-GLP-1 on Parkinson’s disease via inhibiting ferroptosis. Nutrients 14, 4886.
Zarrinpar, A., Chaix, A., Xu, Z.Z., Chang, M.W., Marotz, C.A., Saghatelian, A., Knight, R., and Panda, S. (2018). Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun 9, 2872.
Zhang, C., Fang, R., Lu, X., Zhang, Y., Yang, M., Su, Y., Jiang, Y., and Man, C. (2022a). Lactobacillus reuteri J1 prevents obesity by altering the gut microbiota and regulating bile acid metabolism in obese mice. Food Funct 13, 6688–6701.
Zhang, D., Gao, X., Li, H., Borger, D.K., Wei, Q., Yang, E., Xu, C., Pinho, S., and Frenette, P.S. (2022b). The microbiota regulates hematopoietic stem cell fate decisions by controlling iron availability in bone marrow. Cell Stem Cell 29, 232–247.e7.
Zhang, J., Chen, X., Hong, J., Tang, A., Liu, Y., Xie, N., Nie, G., Yan, X., and Liang, M. (2021). Biochemistry of mammalian ferritins in the regulation of cellular iron homeostasis and oxidative responses. Sci China Life Sci 64, 352–362.
Zhang, J., Zhang, F., Zhao, C., Xu, Q., Liang, C., Yang, Y., Wang, H., Shang, Y., Wang, Y., Mu, X., et al. (2019a). Dysbiosis of the gut microbiome is associated with thyroid cancer and thyroid nodules and correlated with clinical index of thyroid function. Endocrine 64, 564–574.
Zhang, X., Zhu, X., Cao, Y., Fang, J.Y., Hong, J., and Chen, H. (2019b). Fecal Fusobacterium nucleatum for the diagnosis of colorectal tumor: A systematic review and meta-analysis. Cancer Med 8, 480–491.
Zhang, Y., Tan, H., Daniels, J.D., Zandkarimi, F., Liu, H., Brown, L.M., Uchida, K., O’Connor, O.A., and Stockwell, B.R. (2019c). Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol 26, 623–633.e9.
Zheng, P., Zeng, B., Liu, M., Chen, J., Pan, J., Han, Y., Liu, Y., Cheng, K., Zhou, C., Wang, H., et al. (2019). The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv 5, eaau8317.
Zhou, H., Yu, B., Sun, J., Liu, Z., Chen, H., Ge, L., and Chen, D. (2021). Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J Anim Sci Biotechnol 12, 61.
Zhu, Q., Gao, R., Zhang, Y., Pan, D., Zhu, Y., Zhang, X., Yang, R., Jiang, R., Xu, Y., and Qin, H. (2018). Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol Genomics 50, 893–903.
Acknowledgements
This work was supported by Guangdong Basic and Applied Basic Research Foundation (2020B1515020046),“GDAS” Project of Science and Technology Development (2021GDASYL-20210102003), the National Natural Science Foundation of China (81900797, 82072436), and State Key Laboratory of Applied Microbiology Southern China (SKLAM002-2020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xiao, L., Tang, R., Wang, J. et al. Gut microbiota bridges the iron homeostasis and host health. Sci. China Life Sci. 66, 1952–1975 (2023). https://doi.org/10.1007/s11427-022-2302-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-022-2302-5