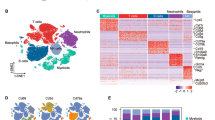
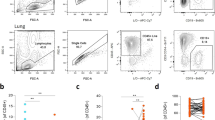
Abstract
Bubonic plague caused by Yersinia pestis is highly infectious and often fatal. Characterization of the host immune response and its subsequent suppression by Y. pestis is critical to understanding the pathogenesis of Y. pestis. Here, we utilized single-cell RNA sequencing to systematically profile the transcriptomes of immune cells in draining lymph nodes (dLNs) during the early stage of Y. pestis infection. Dendritic cells responded to Y. pestis within 2 h post-infection (hpi), followed by the activation of macrophages/monocytes (Mφs/Mons) and recruitment of polymorphonuclear neutrophils (PMNs) to dLNs at 24 hpi. Analysis of cell-to-cell communication suggests that PMNs may be recruited to lymph nodes following the secretion of CCL9 by Mφs/Mons stimulated through CCR1-CCL9 interaction. Significant functional suppression of all the three innate immune cell types occurred during the early stage of infection. In summary, we present a dynamic immune landscape, at single-cell resolution, of murine dLNs involved in the response to Y. pestis infection, which may facilitate the understanding of the plague pathogenesis of during the early stage of infection.
Similar content being viewed by others
References
Berberich, C., Ramírez-Pineda, J.R., Hambrecht, C., Alber, G., Skeiky, Y. A.W., and Moll, H. (2003). Dendritic cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. J Immunol 170, 3171–3179.
Bi, Y., Zhou, J., Yang, H., Wang, X., Zhang, X., Wang, Q., Wu, X., Han, Y., Song, Y., Tan, Y., et al. (2014). IL-17A produced by neutrophils protects against pneumonic plague through orchestrating IFN-γ-activated macrophage programming. J Immunol 192, 704–713.
Bohn, E., Sonnabend, M., Klein, K., and Autenrieth, I.B. (2019). Bacterial adhesion and host cell factors leading to effector protein injection by type III secretion system. Int J Med Microbiol 309, 344–350.
Browaeys, R., Saelens, W., and Saeys, Y. (2020). NicheNet: modeling intercellular communication by linking ligands to target genes. Nat Methods 17, 159–162.
Butler, A., Hoffman, P., Smibert, P., Papalexi, E., and Satija, R. (2018). Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36, 411–420.
Cavanaugh, D.C., and Randall, R. (1959). The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol 83, 348–363.
Chan, G., Bivins-Smith, E.R., Smith, M.S., and Yurochko, A.D. (2009). NF-κB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Res 144, 329–333.
Comer, J.E., Sturdevant, D.E., Carmody, A.B., Virtaneva, K., Gardner, D., Long, D., Rosenke, R., Porcella, S.F., and Hinnebusch, B.J. (2010). Transcriptomic and innate immune responses to Yersinia pestis in the lymph node during bubonic plague. Infect Immun 78, 5086–5098.
Connor, M.G., Pulsifer, A.R., Chung, D., Rouchka, E.C., Ceresa, B.K., and Lawrenz, M.B. (2018). Yersinia pestis targets the host endosome recycling pathway during the biogenesis of the Yersinia-containing vacuole to avoid killing by macrophages. mBio 9.
Deguchi, A., Tomita, T., Omori, T., Komatsu, A., Ohto, U., Takahashi, S., Tanimura, N., Akashi-Takamura, S., Miyake, K., and Maru, Y. (2013). Serum amyloid A3 binds MD-2 to activate p38 and NF-κB pathways in a MyD88-dependent manner. J Immunol 191, 1856–1864.
Du, Z., Yang, H., Tan, Y., Tian, G., Zhang, Q., Cui, Y., Yanfeng Yan, Y., Wu, X., Chen, Z., Cao, S., et al. (2014). Transcriptomic response to Yersinia pestis: RIG-I like receptor signaling response is detrimental to the host against plague. J Genet Genom 41, 379–396.
Frittoli, E., Matteoli, G., Palamidessi, A., Mazzini, E., Maddaluno, L., Disanza, A., Yang, C., Svitkina, T., Rescigno, M., and Scita, G. (2011). The signaling adaptor Eps8 is an essential actin capping protein for dendritic cell migration. Immunity 35, 388–399.
Garrido, D., Alber, A., Kut, E., Chanteloup, N.K., Lion, A., Trotereau, A., Dupont, J., Tedin, K., Kaspers, B., Vervelde, L., et al. (2018). The role of type I interferons (IFNs) in the regulation of chicken macrophage inflammatory response to bacterial challenge. Dev Comp Immunol 86, 156–170.
Gonzalez, R.J., Lane, M.C., Wagner, N.J., Weening, E.H., and Miller, V.L. (2015). Dissemination of a highly virulent pathogen: tracking the early events that define infection. PLoS Pathog 11, e1004587.
Jessen, D.L., Osei-Owusu, P., Toosky, M., Roughead, W., Bradley, D.S., and Nilles, M.L. (2014). Type III secretion needle proteins induce cell signaling and cytokine secretion via Toll-like receptors. Infect Immun 82, 2300–2309.
Kharchenko, P.V., Silberstein, L., and Scadden, D.T. (2014). Bayesian approach to single-cell differential expression analysis. Nat Methods 11, 740–742.
Locati, M., Curtale, G., and Mantovani, A. (2020). Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol Mech Dis 15, 123–147.
Lukaszewski, R.A., Kenny, D.J., Taylor, R., Rees, D.G.C., Hartley, M.G., and Oyston, P.C.F. (2005). Pathogenesis of Yersinia pestis Infection in BALB/c mice: effects on host macrophages and neutrophils. Infect Immun 73, 7142–7150.
Marketon, M.M., DePaolo, R.W., DeBord, K.L., Jabri, B., and Schneewind, O. (2005). Plague bacteria target immune cells during infection. Science 309, 1739–1741.
Mège, J.L., Mehraj, V., and Capo, C. (2011). Macrophage polarization and bacterial infections. Curr Opin Infect Dis 24, 230–234.
Merritt, P.M., Nero, T., Bohman, L., Felek, S., Krukonis, E.S., and Marketon, M.M. (2015). Yersinia pestis targets neutrophils via complement receptor 3. Cell Microbiol 17, 666–687.
Papalexi, E., and Satija, R. (2018). Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol 18, 35–45.
Petri, B., and Sanz, M.J. (2018). Neutrophil chemotaxis. Cell Tissue Res 371, 425–436.
Pujol, C., Grabenstein, J.P., Perry, R.D., and Bliska, J.B. (2005). Replication of Yersinia pestis in interferon γ-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proc Natl Acad Sci USA 102, 12909–12914.
Revollo, I., Nishiura, H., Shibuya, Y., Oda, Y., Nishino, N., and Yamamoto, T. (2005). Agonist and antagonist dual effect of the cross-linked S19 ribosomal protein dimer in the C5a receptor-mediated respiratory burst reaction of phagocytic leukocytes. Inflamm res 54, 82–90.
Ryan, K.J., and Ray, C.G. (2004). Sherris Medical Microbiology (New York: McGraw-Hill).
Sakuma, Y., Kodama, Y., Eguchi, T., Uza, N., Tsuji, Y., Shiokawa, M., Maruno, T., Kuriyama, K., Nishikawa, Y., Yamauchi, Y., et al. (2018). Chemokine CXCL16 mediates acinar cell necrosis in cerulein induced acute pancreatitis in mice. Sci Rep 8, 8829.
Saliba, A.E., Li, L., Westermann, A.J., Appenzeller, S., Stapels, D.A.C., Schulte, L.N., Helaine, S., and Vogel, J. (2016). Single-cell RNA-seq ties macrophage polarization to growth rate of intracellular Salmonella. Nat Microbiol 2, 16206.
Shannon, J.G., Bosio, C.F., and Hinnebusch, B.J. (2015). Dermal neutrophil, macrophage and dendritic cell responses to Yersinia pestis transmitted by fleas. PLoS Pathog 11, e1004734.
Shapouri-Moghaddam, A., Mohammadian, S., Vazini, H., Taghadosi, M., Esmaeili, S.A., Mardani, F., Seifi, B., Mohammadi, A., Afshari, J.T., and Sahebkar, A. (2018). Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233, 6425–6440.
Shepardson, K.M., Larson, K., Morton, R.V., Prigge, J.R., Schmidt, E.E., Huber, V.C., and Rynda-Apple, A. (2016). Differential type I interferon signaling is a master regulator of susceptibility to postinfluenza bacterial superinfection. mBio 7, e00506.
Sing, A., Rost, D., Tvardovskaia, N., Roggenkamp, A., Wiedemann, A., Kirschning, C.J., Aepfelbacher, M., and Heesemann, J. (2002). Yersinia V-antigen exploits toll-like receptor 2 and CD14 for Interleukin 10-mediated immunosuppression. J Exp Med 196, 1017–1024.
Spinner, J.L., Hasenkrug, A.M., Shannon, J.G., Kobayashi, S.D., and Hinnebusch, B.J. (2016). Role of the Yersinia YopJ protein in suppressing interleukin-8 secretion by human polymorphonuclear leukocytes. Microbes Infect 18, 21–29.
Spinner, J.L., Winfree, S., Starr, T., Shannon, J.G., Nair, V., Steele-Mortimer, O., and Hinnebusch, B.J. (2014). Yersinia pestis survival and replication within human neutrophil phagosomes and uptake of infected neutrophils by macrophages. J Leukocyte Biol 95, 389–398.
St. John, A.L., Ang, W.X.G., Huang, M.N., Kunder, C.A., Chan, E.W., Gunn, M.D., and Abraham, S.N. (2014). S1P-Dependent trafficking of intracellular yersinia pestis through lymph nodes establishes Buboes and systemic infection. Immunity 41, 440–450.
Stasulli, N.M., Eichelberger, K.R., Price, P.A., Pechous, R.D., Montgomery, S.A., Parker, J.S., and Goldman, W.E. (2015). Spatially distinct neutrophil responses within the inflammatory lesions of pneumonic plague. mBio 6, e01530.
Stuart, T., Butler, A., Hoffman, P., Hafemeister, C., Papalexi, E., Mauck Iii, W.M., Hao, Y., Stoeckius, M., Smibert, P., and Satija, R. (2019). Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21.
Trapnell, C., Cacchiarelli, D., Grimsby, J., Pokharel, P., Li, S., Morse, M., Lennon, N.J., Livak, K.J., Mikkelsen, T.S., and Rinn, J.L. (2014). The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32, 381–386.
Tuladhar, S., and Kanneganti, T.D. (2020). NLRP12 in innate immunity and inflammation. Mol Aspects Med 76, 100887.
van Rijn, A., Paulis, L., te Riet, J., Vasaturo, A., Reinieren-Beeren, I., van der Schaaf, A., Kuipers, A.J., Schulte, L.P., Jongbloets, B.C., Pasterkamp, R.J., et al. (2016). Semaphorin 7A promotes chemokine-driven dendritic cell migration. J Immunol 196, 459–468.
Vento-Tormo, R., Efremova, M., Botting, R.A., Turco, M.Y., Vento-Tormo, M., Meyer, K.B., Park, J.E., Stephenson, E., Polański, K., Goncalves, A., et al. (2018). Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563, 347–353.
Wei, T., Gong, J., Qu, G., Wang, M., and Xu, H. (2019). Interactions between Yersinia pestis V-antigen (LcrV) and human Toll-like receptor 2 (TLR2) in a modelled protein complex and potential mechanistic insights. BMC Immunol 20, 48.
Xie, X., Shi, Q., Wu, P., Zhang, X., Kambara, H., Su, J., Yu, H., Park, S.Y., Guo, R., Ren, Q., et al. (2020). Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol 21, 1119–1133.
Yamakita, Y., Matsumura, F., Lipscomb, M.W., Chou, P., Werlen, G., Burkhardt, J.K., and Yamashiro, S. (2011). Fascin1 promotes cell migration of mature dendritic cells. J Immunol 186, 2850–2859.
Yamanaka, H., Hoyt, T., Bowen, R., Yang, X., Crist, K., Golden, S., Maddaloni, M., and Pascual, D.W. (2009). An IL-12 DNA vaccine co-expressing Yersinia pestis antigens protects against pneumonic plague. Vaccine 27, 80–87.
Yamanaka, H., Hoyt, T., Yang, X., Golden, S., Bosio, C.M., Crist, K., Becker, T., Maddaloni, M., and Pascual, D.W. (2008). A NASAL Interleukin-12 DNA vaccine coexpressing Yersinia pestis F1–V fusion protein confers protection against pneumonic plague. Infect Immun 76, 4564–4573.
Yang, H., Wang, T., Tian, G., Zhang, Q., Wu, X., Xin, Y., Yan, Y., Tan, Y., Cao, S., Liu, W., et al. (2017). Host transcriptomic responses to pneumonic plague reveal that Yersinia pestis inhibits both the initial adaptive and innate immune responses in mice. Int J Med Microbiol 307, 64–74.
Yu, G., Wang, L.G., Han, Y., and He, Q.Y. (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS-J Integrative Biol 16, 284–287.
Zhang, Q., Wang, Q., Tian, G., Qi, Z., Zhang, X., Wu, X., Qiu, Y., Bi, Y., Yang, X., Xin, Y., et al. (2014). Yersinia pestis biovar Microtus strain 201, an avirulent strain to humans, provides protection against bubonic plague in rhesus macaques. Hum Vaccines Immunother 10, 368–377.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Compliance and ethics
The author(s) declare that they have no conflict of interest.
Supplementary materials
Rights and permissions
About this article
Cite this article
Zhao, Y., Wang, T., Liu, Z. et al. Single-cell transcriptomics of immune cells in lymph nodes reveals their composition and alterations in functional dynamics during the early stages of bubonic plague. Sci. China Life Sci. 66, 110–126 (2023). https://doi.org/10.1007/s11427-021-2119-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-021-2119-5