Abstract
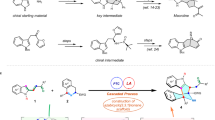
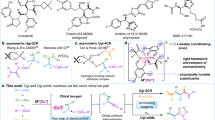
A privileged strategy has been developed for the precise construction of enantioenriched azaspiro polycyclic scaffolds. Structurally diverse azaspiro polycycles bearing multiple contiguous stereocenters are obtained with excellent results (up to 99:1 e.r., >95:5 d.r.) via sequential enantioselective four-component Ugi reactions/post-Ugi transformations with substrates containing prerequisite functional groups in the presence of anionic stereogenic-at-cobalt(III) complexes.

Similar content being viewed by others
References
Biard JF, Guyot S, Roussakis C, Verbist JF, Vercauteren J, Weber JF, Boukef K. Tetrahedron Lett, 1994, 35: 2691–2694
Hesse M, Ünver N, Noyan S, Gözler B, Gözler T, Werner C. Heterocycles, 2001, 55: 641–652
Verbitski SM, Mayne CL, Davis RA, Concepcion GP, Ireland CM. J Org Chem, 2002, 67: 7124–7126
Masi M, Di Lecce R, Cimmino A, Evidente A. Molecules, 2020, 25: 5621
Jin Z, Yao G. Nat Prod Rep, 2019, 36: 1462–1488
Jin Z. Nat Prod Rep, 2016, 33: 1318–1343
Trost BM, Osipov M. Chem Eur J, 2015, 21: 16318–16343
Baxendale IR, Ley SV, Piutti C. Angew Chem Int Ed, 2002, 41: 2194–2197
Baxendale IR, Ley SV, Nessi M, Piutti C. Tetrahedron, 2002, 58: 6285–6304
Takeuchi H, Inuki S, Nakagawa K, Kawabe T, Ichimura A, Oishi S, Ohno H. Angew Chem Int Ed, 2020, 59: 21210–21215
Sharma UK, Ranjan P, Van der Eycken EV, You SL. Chem Soc Rev, 2020, 49: 8721–8748
Nunes PSG, Vidal HDA, Corrêa AG. Org Biomol Chem, 2020, 18: 7751–7773
Ruijter E, Scheffelaar R, Orru RVA. Angew Chem Int Ed, 2011, 50: 6234–6246
Neto BAD, Rocha RO, Rodrigues MO. Molecules, 2022, 27: 132
Ramón DJ, Yus M. Angew Chem Int Ed, 2005, 44: 1602–1634
Paulvannan K. Tetrahedron Lett, 1999, 40: 1851–1854
Lee D, Sello JK, Schreiber SL. Org Lett, 2000, 2: 709–712
Paulvannan K. J Org Chem, 2004, 69: 1207–1214
Santra S, Andreana PR. Org Lett, 2007, 9: 5035–5038
Santra S, Andreana PR. Angew Chem Int Ed, 2011, 50: 9418–9422
Modha SG, Kumar A, Vachhani DD, Jacobs J, Sharma SK, Parmar VS, Van Meervelt L, Van der Eycken EV. Angew Chem Int Ed, 2012, 51: 9572–9575
Yugandhar D, Kuriakose S, Nanubolu JB, Srivastava AK. Org Lett, 2016, 18: 1040–1043
Mijangos MV, Miranda LD. Org Biomol Chem, 2016, 14: 3677–3680
He Y, Li Z, Tian G, Song L, Van Meervelt L, Van der Eycken EV. Chem Commun, 2017, 53: 6413–6416
He Y, Li Z, Robeyns K, Van Meervelt L, Van der Eycken EV. Angew Chem Int Ed, 2018, 57: 272–276
He Y, Song L, Liu C, Wu D, Li Z, Van Meervelt L, Van der Eycken EV. J Org Chem, 2020, 85: 15092–15103
Singh SP, Tripathi S, Yadav A, Kant R, Srivastava HK, Srivastava AK. Chem Commun, 2020, 56: 12789–12792
Zhang J, Yu P, Li SY, Sun H, Xiang SH, Wang JJ, Houk KN, Tan B. Science, 2018, 361: eaas8707
Sun BB, Liu K, Gao Q, Fang W, Lu S, Wang CR, Yao CZ, Cao HQ, Yu J. Nat Commun, 2022, 13: 7065
Wang Q, Wang DX, Wang MX, Zhu J. Acc Chem Res, 2018, 51: 1290–1300
Luo J, Chen G, Chen S, Li Z, Liu Y. Chem Eur J, 2021, 27: 6598–6619
Flores-Reyes JC, Islas-Jácome A, González-Zamora E. Org Chem Front, 2021, 8: 5460–5515
Pan SC, List B. Angew Chem Int Ed, 2008, 47: 3622–3625
Yue T, Wang MX, Wang DX, Masson G, Zhu J. Angew Chem Int Ed, 2009, 48: 6717–6721
Hashimoto T, Kimura H, Kawamata Y, Maruoka K. Angew Chem Int Ed, 2012, 51: 7279–7281
Zhao W, Huang L, Guan Y, Wulff WD. Angew Chem Int Ed, 2014, 53: 3436–3441
Zhang Y, Ao Y, Huang Z, Wang D, Wang M, Zhu J. Angew Chem Int Ed, 2016, 55: 5282–5285
Feng QY, Zhu J, Wang MX, Tong S. Org Lett, 2020, 22: 483–487
Zhang J, Wang YY, Sun H, Li SY, Xiang SH, Tan B. Sci China Chem, 2020, 63: 47–54
Belokon YN, Belikov VM, Vitt SV, Saveleva TF, Burbelo VM, Bakhmutov VI, Aleksandrov GG, Struchkov YT. Tetrahedron, 1977, 33: 2551–2564
Belokon YN, Bulychev AG, Maleev VI, North M, Malfanov IL, Ikonnikov NS. Mendeleev Commun, 2004, 14: 249–250
Belokon YN, Maleev VI, Mal’fanov IL, Savel’eva TF, Ikonnikov NS, Bulychev AG, Usanov DL, Kataev DA, North M. Russ Chem Bull, 2006, 55: 821–827
Belokon YN, Maleev VI, Kataev DA, Mal’fanov IL, Bulychev AG, Moskalenko MA, Saveleva TF, Skrupskaya TV, Lyssenko KA, Godovikov IA, North M. Tetrahedron-Asymmetry, 2008, 19: 822–831
Belokon YN, Maleev VI, Kataev DA, Saveleva TF, Skrupskaya TV, Nelyubina YV, North M. Tetrahedron-Asymmetry, 2009, 20: 1746–1752
Maleev VI, Skrupskaya TV, Yashkina LV, Mkrtchyan AF, Saghyan AS, Il’in MM, Chusov DA. Tetrahedron-Asymmetry, 2013, 24: 178–183
Yu J, Jiang HJ, Zhou Y, Luo SW, Gong LZ. Angew Chem Int Ed, 2015, 54: 11209–11213
Jiang H, Liu K, Yu J, Zhang L, Gong L. Angew Chem Int Ed, 2017, 56: 11931–11935
Wu XB, Gao Q, Fan JJ, Zhao ZY, Tu XQ, Cao HQ, Yu J. Org Lett, 2021, 23: 9134–9139
Jiang H, Zhong X, Yu J, Zhang Y, Zhang X, Wu Y, Gong L. Angew Chem Int Ed, 2019, 58: 1803–1807
Jiang H, Geng R, Wei J, Gong L. Chin J Chem, 2021, 39: 3269–3276
Zhang X, Zhao K, Li N, Yu J, Gong L, Gu Z. Angew Chem Int Ed, 2020, 59: 19899–19904
Liu YZ, Song H, Zheng C, You SL. Nat Synth, 2022, 1: 203–216
Zheng C, You SL. ACS Cent Sci, 2021, 7: 432–444
An J, Bandini M. Eur J Org Chem, 2020, 27: 4087–4097
Zheng C, You SL. Chem, 2016, 1: 830–857
Wu WT, Zhang L, You SL. Chem Soc Rev, 2016, 45: 1570–1580
Zhao K, Kohnke P, Yang Z, Cheng X, You S, Zhang L. Angew Chem Int Ed, 2022, 61: e202207518
Ding L, Wu WT, Zhang L, You SL. Org Lett, 2020, 22: 5861–5865
An J, Lombardi L, Grilli S, Bandini M. Org Lett, 2018, 20: 7380–7383
Wu WT, Xu RQ, Zhang L, You SL. Chem Sci, 2016, 7: 3427–3431
Aparece MD, Vadola PA. Org Lett, 2014, 16: 6008–6011
Nemoto T, Matsuo N, Hamada Y. Adv Synth Catal, 2014, 356: 2417–2421
Oka J, Okamoto R, Noguchi K, Tanaka K. Org Lett, 2015, 17: 676–679
Vacala TL, Carlson PR, Arreola-Hester A, Williams CG, Makhoul EW, Vadola PA. J Org Chem, 2018, 83: 1493–1501
Wang H, Wang K, Xiang Y, Jiang H, Wan X, Li N, Tang B. Adv Synth Catal, 2018, 360: 2352–2357
Ito M, Kawasaki R, Kanyiva KS, Shibata T. Chem Eur J, 2018, 24: 3721–3724
Wu WT, Ding L, Zhang L, You SL. Org Lett, 2020, 22: 1233–1238
Li Y, Zhao X. Catal Sci Technol, 2020, 10: 2415–2426
See Supporting Information for details
Sharma UK, Sharma N, Vachhani DD, Van der Eycken EV. Chem Soc Rev, 2015, 44: 1836–1860
Acknowledgements
This work was supported by the National Natural Science Foundation of China (92156022), the Anhui Provincial Natural Science Funds (1908085J07, 1908085QB79, 2308085MB44, 2308085QB44), and the Shen-Nong Scholar Program of Anhui Agricultural University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at chem.scichina.com and link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supplemental Information for
11426_2023_1782_MOESM1_ESM.pdf
Sequential Enantioselective Ugi-4CR/post-Ugi Transformation Strategy: A Precise Construction of Structurally Diverse Azaspiro Polycyclic Scaffolds
Rights and permissions
About this article
Cite this article
Wu, XB., Shi, JX., Ou, YM. et al. Sequential enantioselective Ugi-4CR/post-Ugi transformation strategy: a precise construction of structurally diverse azaspiro polycyclic scaffolds. Sci. China Chem. 67, 576–586 (2024). https://doi.org/10.1007/s11426-023-1782-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1782-9