Abstract
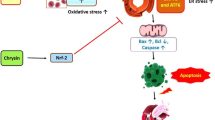
Corilagin, a natural polyphenol compound isolated from Phyllanthus urinaria L., exerts various pharmacological effects, such as antihyperglycemic, antitumor, and antioxidative stress properties, but the mechanisms underlying the antiatherosclerotic effects of corilagin have not been entirely elucidated. In the present study, we investigated the antiatherosclerotic effects of corilagin using a high-fat diet (HFD)-induced atherosclerotic rabbit model and ox-LDL-induced vascular smooth muscle cells (VSMCs) and explored the underlying molecular mechanisms. The serum lipid levels were measured through an enzymatic colorimetric assay. A histological analysis of rabbit aortas was performed after hematoxylin–eosin and oil red O staining. The proliferation of ox-LDL-induced VSMCs was detected using MTT assays, and the migration of cells was determined by wound scratch assays. In addition, the mRNA and protein expression levels of lectin-like ox-LDL receptor-1 (LOX-1), myeloid differentiation factor 88 (MyD88), nuclear factor-kappa B (NF-κB), monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor α (TNF-α) were detected by reverse transcription-polymerase chain reaction (RT–PCR) and Western blotting assays. Our results indicate that corilagin significantly reduced the serum levels of TC, TG and LDL-C, increased the HDL-C levels, decreased the intimal thickening in the thoracic aorta, and reduced the formation of foam cells in an HFD-induced rabbit atherosclerosis model. Moreover, corilagin suppressed the proliferation and migration of ox-LDL-induced VSMCs and reduced LOX-1, MyD88, NF-κB, MCP-1, and TNF-α mRNA and protein expression in vivo and in vitro. These data demonstrate that corilagin exerts antiatherosclerotic effects in vivo and in vitro and that the mechanisms may be closely associated with downregulation of the LOX-1/MyD88/NF-κB pathway.
Graphical abstract









Similar content being viewed by others
References
Kurkowska-Jastrzebska I, Karlinski MA, Blazejewska-Hyzorek B, Sarzynska-Dlugosz I, Filipiak KJ, Czlonkowska A (2016) Carotid intima media thickness and blood biomarkers of atherosclerosis in patients after stroke or myocardial infarction. Croat Med J 57:548–557. https://doi.org/10.3325/cmj.2016.57.548
Sedding DG, Boyle EC, JaF D, Sluimer JC, Dutzmann J, Haverich A, Bauersachs J (2018) Vasa vasorum angiogenesis: key player in the initiation and progression of atherosclerosis and potential target for the treatment of cardiovascular disease. Front Immunol 9:706. https://doi.org/10.3389/fimmu.2018.00706
Wang C, Niimi M, Watanabe T, Wang Y, Liang J, Fan J (2018) Treatment of atherosclerosis by traditional Chinese medicine: questions and quandaries. Atherosclerosis 277:136–144. https://doi.org/10.1016/j.atherosclerosis.2018.08.039
Li X, Zhang Y, Wang Y, Xu J, Xin P, Meng Y, Wang Q, Kuang H (2017) The mechanisms of traditional Chinese medicine underlying the prevention and treatment of Parkinson’s disease. Front Pharmacol 8:634. https://doi.org/10.3389/fphar.2017.00634
Wang M, Wu Y, Yu Y, Fu Y, Yan H, Wang X, Li T, Peng W, Luo D (2019) Rutaecarpine prevented ox-LDL-induced VSMCs dysfunction through inhibiting overexpression of connexin 43. Eur J Pharmacol 853:84–92. https://doi.org/10.1016/j.ejphar.2019.03.028
Suzuki T, Chiang HJ, Yanaura S, Yoshida T, Kuroiwa Y (1986) Studies on the mechanism of interaction between methamphetamine and quinine in rats. J Pharmacobiodyn 9:234–238. https://doi.org/10.1248/bpb1978.9.234
Kattoor AJ, Kanuri SH, Mehta JL (2019) Role of Ox-LDL and LOX-1 in atherogenesis. Curr Med Chem 26:1693–1700. https://doi.org/10.2174/0929867325666180508100950
Chen T, Luo W, Wu G, Wu L, Huang S, Li J, Wang J, Hu X, Huang W, Liang G (2019) A novel MyD88 inhibitor LM9 prevents atherosclerosis by regulating inflammatory responses and oxidative stress in macrophages. Toxicol Appl Pharmacol 370:44–55. https://doi.org/10.1016/j.taap.2019.03.012
Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M (2004) Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101:10679–10684. https://doi.org/10.1073/pnas.0403249101
Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schroder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M (2008) TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol 181:7176–7185. https://doi.org/10.4049/jimmunol.181.10.7176
Liang X, Xiu C, Liu M, Lin C, Chen H, Bao R, Yang S, Yu J (2019) Platelet-neutrophil interaction aggravates vascular inflammation and promotes the progression of atherosclerosis by activating the TLR4/NF-kappaB pathway. J Cell Biochem 120:5612–5619. https://doi.org/10.1002/jcb.27844
An J, Li Z, Dong Y, Ren J, Guo K (2016) Methicillin-resistant staphylococcus aureus infection exacerbates NSCLC cell metastasis by up-regulating TLR4/MyD88 pathway. Cell Mol Biol (Noisy-le-grand) 62:1–7
Nandini HS, Naik PR (2019) Action of corilagin on hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. Chem Biol Interact 299:186–193. https://doi.org/10.1016/j.cbi.2018.12.012
Xu J, Zhang G, Tong Y, Yuan J, Li Y, Song G (2019) Corilagin induces apoptosis, autophagy and ROS generation in gastric cancer cells in vitro. Int J Mol Med 43:967–979. https://doi.org/10.3892/ijmm.2018.4031
Ding Y, Ren D, Xu H, Liu W, Liu T, Li L, Li J, Li Y, Wen A (2017) Antioxidant and pro-angiogenic effects of corilagin in rat cerebral ischemia via Nrf2 activation. Oncotarget 8:114816–114828. https://doi.org/10.18632/oncotarget.22023
Shen ZQ, Dong ZJ, Peng H, Liu JK (2003) Modulation of PAI-1 and tPA activity and thrombolytic effects of corilagin. Planta Med 69:1109–1112. https://doi.org/10.1055/s-2003-45191
Wu YX, Dong YW, Liu Y, Peng YG, Wang L, Sheng ZQ (2009) Effect of corilagin on thrombosis and its mechanisms. Chinese J New Drugs 18:1008–1011
Tao Y, Zhang L, Yang R, Yang Y, Jin H, Zhang X, Hu Q, He B, Shen Z, Chen P (2021) Corilagin ameliorates atherosclerosis by regulating MMP-1, -2, and -9 expression in vitro and in vivo. Eur J Pharmacol 906:174200. https://doi.org/10.1016/j.ejphar.2021.174200
Li Y, Wang Y, Chen Y, Wang Y, Zhang S, Liu P, Chen Z, Song P, Luo L, Luo Y, Dang Y, Zhao L (2020) Corilagin ameliorates atherosclerosis in peripheral artery disease via the toll-like receptor-4 signaling pathway in vitro and in vivo. Front Immunol 11:1611. https://doi.org/10.3389/fimmu.2020.01611
Ulrich-Merzenich G, Zeitler H (2013) The lectin-like oxidized low-density lipoprotein receptor-1 as therapeutic target for atherosclerosis, inflammatory conditions and longevity. Expert Opin Ther Targets 17:905–919. https://doi.org/10.1517/14728222.2013.805748
Gupta RL, Bhatnagar VM, Seth S (1970) Some observations with rolitetracycline as a vasodilator. Indian J Med Res 58:83–86
Chen Z, Pan X, Sheng Z, Yan G, Chen L, Ma G (2019) Baicalin suppresses the proliferation and migration of Ox-LDL-VSMCs in atherosclerosis through upregulating miR-126-5p. Biol Pharm Bull 42:1517–1523. https://doi.org/10.1248/bpb.b19-00196
Lechner K, Mckenzie AL, Krankel N, Von Schacky C, Worm N, Nixdorff U, Lechner B, Scherr J, Weingartner O, Krauss RM (2020) High-risk atherosclerosis and metabolic phenotype: the roles of ectopic adiposity, atherogenic dyslipidemia, and inflammation. Metab Syndr Relat Disord 18:176–185. https://doi.org/10.1089/met.2019.0115
Oikonomidis N, Kavantzas N, Korou LM, Konstantopoulos P, Pergialiotis V, Misiakos E, Rizos I, Verikokos C, Perrea DN (2016) Pre-treatment with simvastatin prevents the induction of diet-induced atherosclerosis in a rabbit model. Biomed Rep 5:667–674. https://doi.org/10.3892/br.2016.780
Wang W, Chen Y, Bai L, Zhao S, Wang R, Liu B, Zhang Y, Fan J, Liu E (2018) Transcriptomic analysis of the liver of cholesterol-fed rabbits reveals altered hepatic lipid metabolism and inflammatory response. Sci Rep 8:6437. https://doi.org/10.1038/s41598-018-24813-1
Korou LM, Agrogiannis G, Koros C, Kitraki E, Vlachos IS, Tzanetakou I, Karatzas T, Pergialiotis V, Dimitroulis D, Perrea DN (2014) Impact of N-acetylcysteine and sesame oil on lipid metabolism and hypothalamic-pituitary-adrenal axis homeostasis in middle-aged hypercholesterolemic mice. Sci Rep 4:6806. https://doi.org/10.1038/srep06806
Yanni AE (2004) The laboratory rabbit: an animal model of atherosclerosis research. Lab Anim 38:246–256. https://doi.org/10.1258/002367704323133628
Potor L, Eva SK, Hegedus H, Petho D, Szabo Z, Szigeti ZM, Pocsi I, Trencsenyi G, Szikra D, Garai I, Gall T, Combi Z, Kappelmayer J, Balla G, Balla J (2020) The fungal iron chelator desferricoprogen inhibits atherosclerotic plaque formation. Int J Mol Sci 21:4746. https://doi.org/10.3390/ijms21134746
Goetz TG, Mamillapalli R, Sahin C, Majidi-Zolbin M, Ge G, Mani A, Taylor HS (2018) Addition of estradiol to cross-sex testosterone therapy reduces atherosclerosis plaque formation in female ApoE-/- mice. Endocrinology 159:754–762. https://doi.org/10.1210/en.2017-00884
Li HX, Han M, Bernier M, Zheng B, Sun SG, Su M, Zhang R, Fu JR, Wen JK (2010) Kruppel-like factor 4 promotes differentiation by transforming growth factor-beta receptor-mediated Smad and p38 MAPK signaling in vascular smooth muscle cells. J Biol Chem 285:17846–17856. https://doi.org/10.1074/jbc.M109.076992
Zhou Y, Zhang MJ, Li BH, Chen L, Pi Y, Yin YW, Long CY, Wang X, Sun MJ, Chen X, Gao CY, Li JC, Zhang LL (2016) PPARgamma inhibits VSMC proliferation and migration via attenuating oxidative stress through upregulating UCP2. PLoS ONE 11:e0154720. https://doi.org/10.1371/journal.pone.0154720
Sun Y, Chen D, Cao L, Zhang R, Zhou J, Chen H, Li Y, Li M, Cao J, Wang Z (2013) MiR-490-3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A. Cardiovasc Res 100:272–279. https://doi.org/10.1093/cvr/cvt172
Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, Mallat Z (2019) Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol 16:727–744. https://doi.org/10.1038/s41569-019-0227-9
Yan S, Wu T, Li N, Zhang L, Song J, Xu Y, Wang S, Ding L, Jin J, Liu Y, Lan T (2018) Protective effects of Chinese traditional medicine longhu rendan against atherosclerosis via negative regulation of LOX-1. Evid Based Complement Alternat Med 2018:4812639. https://doi.org/10.1155/2018/4812639
Tian K, Ogura S, Little PJ, Xu SW, Sawamura T (2019) Targeting LOX-1 in atherosclerosis and vasculopathy: current knowledge and future perspectives. Ann N Y Acad Sci 1443:34–53. https://doi.org/10.1111/nyas.13984
Geng H, Wang A, Rong G, Zhu B, Deng Y, Chen J, Zhong R (2010) The effects of ox-LDL in human atherosclerosis may be mediated in part via the toll-like receptor 4 pathway. Mol Cell Biochem 342:201–206. https://doi.org/10.1007/s11010-010-0484-8
Xie J, Zhu H, Guo L, Ruan Y, Wang L, Sun L, Zhou L, Wu W, Yun X, Shen A, Gu J (2010) Lectin-like oxidized low-density lipoprotein receptor-1 delivers heat shock protein 60-fused antigen into the MHC class I presentation pathway. J Immunol 185:2306–2313. https://doi.org/10.4049/jimmunol.0903214
Liu T, Wu XZ, Chen JW, Yang J, Shen ZQ, Liu G, Chai WY, Chen P (2020) Effects of corilagin on the expression of TLR4 in atherosclerotic plaques of rabbits and ox-LDL-injured HUVEc. J Kunming Med Univ 41:15–20
Zhu G, Cheng Z, Huang Y, Zheng W, Yang S, Lin C, Ye J (2020) MyD88 mediates colorectal cancer cell proliferation, migration and invasion via NFkappaB/AP1 signaling pathway. Int J Mol Med 45:131–140. https://doi.org/10.3892/ijmm.2019.4390
Yan F, Guan J, Peng Y, Zheng X (2017) MyD88 NEDDylation negatively regulates MyD88-dependent NF-kappaB signaling through antagonizing its ubiquitination. Biochem Biophys Res Commun 482:632–637. https://doi.org/10.1016/j.bbrc.2016.11.084
Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW (2004) Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 10:416–421. https://doi.org/10.1038/nm1008
Bahrami A, Parsamanesh N, Atkin SL, Banach M, Sahebkar A (2018) Effect of statins on toll-like receptors: a new insight to pleiotropic effects. Pharmacol Res 135:230–238. https://doi.org/10.1016/j.phrs.2018.08.014
Koushki K, Shahbaz SK, Mashayekhi K, Sadeghi M, Zayeri ZD, Taba MY, Banach M, Al-Rasadi K, Johnston TP, Sahebkar A (2021) Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin Rev Allergy Immunol 60:175–199. https://doi.org/10.1007/s12016-020-08791-9
Lecarpentier Y, Claes V, Duthoit G, Hebert JL (2014) Circadian rhythms, Wnt/beta-catenin pathway and PPAR alpha/gamma profiles in diseases with primary or secondary cardiac dysfunction. Front Physiol 5:429. https://doi.org/10.3389/fphys.2014.00429
Marz W, Koenig W (2003) HMG-CoA reductase inhibition: anti-inflammatory effects beyond lipid lowering? J Cardiovasc Risk 10:169–179. https://doi.org/10.1097/01.hjr.0000073686.78271.6d
Kvandova M, Majzunova M, Dovinova I (2016) The role of PPARgamma in cardiovascular diseases. Physiol Res 65:S343–S363. https://doi.org/10.33549/physiolres.933439
Lu C, Yin Y, Cui Y, Wang L, Bai Y, Li J, Huang T, Reziwanguli M, Miao L (2019) 1,25(OH)2D3 improves blood lipid metabolism, liver function, and atherosclerosis by constraining the TGF-beta/Smad signaling pathway in rats with hyperlipidemia. Cell Cycle 18:3111–3124. https://doi.org/10.1080/15384101.2019.1669389
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81660613 and 81860665), by the Natural Science Foundation of Yunnan Province, China (Grant Nos. 2019FE001-191 and 202001AY070001-157), and by the Digitalization, Development and Application of Biotic Resources of Kunming Medical University (Grant No. 202002AA100007).
Author information
Authors and Affiliations
Contributions
PC and ZQS: conceptualization; BH and ZQS: methodology; BH, DYC, XCZ, RHY, and YY: validation; BH and DYC: writing—original draft preparation; PC and ZQS: writing—review and editing. All the authors have read and agreed to the submission of this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, B., Chen, D., Zhang, X. et al. Antiatherosclerotic effects of corilagin via suppression of the LOX-1/MyD88/NF-κB signaling pathway in vivo and in vitro. J Nat Med 76, 389–401 (2022). https://doi.org/10.1007/s11418-021-01594-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-021-01594-y