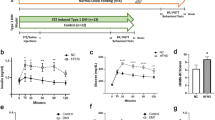
Abstract
Cerebral small vessel diseases (CSVD) are neurological disorders associated with microvessels, manifested pathologically as white matter (WM) changes and cortical microbleeds, with hypertension as a risk factor. Additionally, a high-fat diet (HFD) can affect peripheral vessel health. Our study explored how HFD affects cerebral small vessels in normotensive WKY, hypertensive SHR, and SHR/SP rats. The MRI results revealed that HFD specifically increased WM hyperintensity in SHR/SP rats. Pathologically, it increased WM pallor and vacuolation in SHR and SHR/SP rats. Levels of blood–brain barrier (BBB) protein claudin 5 were decreased in SHR and SHR/SP compared to WKY, with HFD having minimal impact on these levels. Conversely, collagen IV levels remained consistent among the rat strains, which were increased by HFD. Consequently, HFD caused vessel leakage in all rat strains, particularly within the corpus callosum of SHR/SP rats. To understand the underlying mechanisms, we assessed the levels of hypoxia-inducible factor-1α (HIF-1α), Gp91-phox, and neuroinflammatory markers astrocytes, and microglia were increased in SHR and SHR/SP compared to WKY and were further elevated by HFD in all rat strains. Gp91-phox was also increased in SHR and SHR/SP compared to WKY, with HFD causing an increase in WKY but little effect in SHR and SHR/SP. In conclusion, our study demonstrates that HFD, in combined with hypertension, intensifies cerebral pathological alterations in CSVD rats. This exacerbation involves increased oxidative stress and HIF-1α in cerebral vessels, triggering neuroinflammation, vascular basement membrane remodeling, IgG leakage, and ultimately WM damage.
Graphical Abstract










Similar content being viewed by others
Data Availability
The data that support the findings of this study are available upon request from the authors.
References
Chojdak-Łukasiewicz J, Dziadkowiak E, Zimny A, Paradowski B. Cerebral small vessel disease: A review. Adv Clin Exp Med. 2021;30(3):349–56. https://doi.org/10.17219/acem/131216.
Cuadrado-Godia E, Dwivedi P, Sharma S, Ois Santiago A, Roquer Gonzalez J, Balcells M, Laird J, Turk M, Suri HS, Nicolaides A, Saba L, Khanna NN, Suri JS. Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J Stroke. 2018;20(3):302–20. https://doi.org/10.5853/jos.2017.02922.
Li Q, Yang Y, Reis C, Tao T, Li W, Li X, Zhang JH. Cerebral Small Vessel Disease. Cell Transplant. 2018;27(12):1711–22. https://doi.org/10.1177/0963689718795148.
Lee WJ, Chou KH, Lee PL, Peng LN, Wang PN, Lin CP, Chen LK, Chung CP. Cerebral small vessel disease phenotype and 5-year mortality in asymptomatic middle-to-old aged individuals. Sci Rep. 2021;11(1):23149. https://doi.org/10.1038/s41598-021-02656-7.
Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164–73. https://doi.org/10.1016/j.neubiorev.2018.04.00.
Ihara M, Yamamoto Y. Emerging Evidence for Pathogenesis of Sporadic Cerebral Small Vessel Disease. Stroke. 2016;47(2):554–60. https://doi.org/10.1161/STROKEAHA.115.009627.
Heye AK, Thrippleton MJ, Chappell FM, Hernández Mdel C, Armitage PA, Makin SD, Maniega SM, Sakka E, Flatman PW, Dennis MS, Wardlaw JM. Blood pressure and sodium: Association with MRI markers in cerebral small vessel disease. J Cereb Blood Flow Metab. 2016;36(1):264–74. https://doi.org/10.1038/jcbfm.2015.64.
Wang Z, Chen Q, Chen J, Yang N, Zheng K. Risk factors of cerebral small vessel disease: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100(51): e28229. https://doi.org/10.1097/MD.0000000000028229.
Inoue Y, Shue F, Bu G, Kanekiyo T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol Neurodegener. 2023;18(1):46. https://doi.org/10.1186/s13024-023-00640-5.
Marini S, Anderson CD, Rosand J. Genetics of Cerebral Small Vessel Disease. Stroke. 2020;51(1):12–20. https://doi.org/10.1161/STROKEAHA.119.024151.
Bai T, Yu S, Feng J. Advances in the Role of Endothelial Cells in Cerebral Small Vessel Disease. Front Neurol. 2022;13: 861714. https://doi.org/10.3389/fneur.2022.861714.
Rajeev V, Fann DY, Dinh QN, Kim HA, De Silva TM, Lai MKP, Chen CL, Drummond GR, Sobey CG, Arumugam TV. Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics. 2022;12(4):1639–58. https://doi.org/10.7150/thno.68304.
Ohtsuki S, Yamaguchi H, Katsukura Y, Asashima T, Terasaki T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem. 2008;104(1):147–54. https://doi.org/10.1111/j.1471-4159.2007.05008.x.
Dearborn JL, Schneider AL, Sharrett AR, Mosley TH, Bezerra DC, Knopman DS, Selvin E, Jack CR, Coker LH, Alonso A, Wagenknecht LE, Windham BG, Gottesman RF. Obesity, Insulin Resistance, and Incident Small Vessel Disease on Magnetic Resonance Imaging: Atherosclerosis Risk in Communities Study. Stroke. 2015;46(11):3131–6. https://doi.org/10.1161/STROKEAHA.115.010060.
Tb M, G, T., A, G., D, A. Erratum to “Suffering from Cerebral Small Vessel Disease with and without Metabolic Syndrome.” Open Med (Wars). 2020;16(1):23. https://doi.org/10.1515/med-2021-0006.
Nassir CMNCM, Ghazali MM, Hashim S, Idris NS, Yuen LS, Hui WJ, Norman HH, Gau CH, Jayabalan N, Na Y, Feng L, Ong LK, Abdul Hamid H, Ahamed HN, Mustapha M. Diets and Cellular-Derived Microparticles: Weighing a Plausible Link With Cerebral Small Vessel Disease. Front Cardiovasc Med. 2021;8:632131. https://doi.org/10.3389/fcvm.2021.632131.
Chen YC, Lu BZ, Shu YC, Sun YT. Spatiotemporal Dynamics of Cerebral Vascular Permeability in Type 2 Diabetes-Related Cerebral Microangiopathy. Front Endocrinol (Lausanne). 2022;12: 805637. https://doi.org/10.3389/fendo.2021.805637.
Attuquayefio T, Stevenson RJ, Oaten MJ, Francis HM. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS ONE. 2017;12(2): e0172645. https://doi.org/10.1371/journal.pone.0172645.
Clemente-Suárez VJ, Beltrán-Velasco AI, Redondo-Flórez L, Martín-Rodríguez A, Tornero-Aguilera JF. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients. 2023;15(12):2749. https://doi.org/10.3390/nu15122749.
Mustapha M, Nassir CMNCM, Aminuddin N, Safri AA, Ghazali MM. Cerebral Small Vessel Disease (CSVD) - Lessons From the Animal Models. Front Physiol. 2019;10:1317. https://doi.org/10.3389/fphys.2019.01317.
Olivera, S., Graham, D. Sex differences in preclinical models of hypertension. J Hum Hypertens, 2022. 1–7. https://doi.org/10.1038/s41371-022-00770-1
Yamori Y, Horie R, Sato M, Ohta K. Proceedings: Prophylactic trials for stroke in stroke-prone SHR: effect of sex hormones. Jpn Heart J. 1976;17(3):404–6. https://doi.org/10.1536/ihj.17.404.
Tochinai R, Sekizawa S, Kobayashi I, Kuwahara HM. Autonomic nervous activity in rats can be evaluated by blood photoplethysmography-derived pulse rate variability analysis. Transl Regul Sci. 2021;3(1):17–21. https://doi.org/10.33611/trs.2021-001.
Kuwahara M, Sugano S, Yayou K, Tsubone H, Kobayashi H. Evaluation of a new tail-cuff method for blood pressure measurements in rats with special reference to the effects of ambient temperature. Jikken Dobutsu. 1991;40(3):331–6. https://doi.org/10.1538/expanim1978.40.3_331.
Sasaki K, Yoshizaki F. Investigation into hippocampal nerve cell damage through the mineralocorticoid receptor in mice. Mol Med Rep. 2015;12(5):7211–20. https://doi.org/10.3892/mmr.2015.4406.
Herisson F, Zhou I, Mawet J, Du E, Barfejani AH, Qin T, Cipolla MJ, Sun PZ, Rost NS, Ayata C. Posterior reversible encephalopathy syndrome in stroke-prone spontaneously hypertensive rats on high-salt diet. J Cereb Blood Flow Metab. 2019;39(7):1232–46. https://doi.org/10.1177/0271678X17752795.
Azad AK, Sheikh AM, Haque MA, Osago H, Sakai H, Shibly AZ, Yano S, Michikawa M, Hossain S, Tabassum S, Zhou AG, Zhang X, Nagai YA. Time-Dependent Analysis of Plasmalogens in the Hippocampus of an Alzheimer’s Disease Mouse Model: A Role of Ethanolamine Plasmalogen. Brain Sci. 2021;11(12):1603. https://doi.org/10.3390/brainsci11121603.
Shibly AZ, Sheikh AM, Michikawa M, Tabassum S, Azad AK, Zhou X, Zhang Y, Yano S, Nagai A. Analysis of Cerebral Small Vessel Changes in AD Model Mice. Biomedicines. 2022;11(1):50. https://doi.org/10.3390/biomedicines11010050.
Weiss HR, Buchweitz E, Murtha TJ, Auletta M. Quantitative regional determination of morphometric indices of the total and perfused capillary network in the rat brain. Circ Res. 1982;51(4):494–503. https://doi.org/10.1161/01.RES.51.4.494.
Saubaméa B, Cochois-Guégan V, Cisternino S, Scherrmann JM. Heterogeneity in the rat brain vasculature revealed by quantitative confocal analysis of endothelial barrier antigen and P-glycoprotein expression. J Cereb Blood Flow Metab. 2012;32(1):81–92. https://doi.org/10.1038/jcbfm.2011.109.
Lee SP, Duong TQ, Yang G, Iadecola C, Kim SG. Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: implications for BOLD fMRI. Magn Reson Med. 2001;45(5):791–800. https://doi.org/10.1002/mrm.1107.
Elabi OF, Cunha JPMCM, Gaceb A, Fex M, Paul G. High-fat diet-induced diabetes leads to vascular alterations, pericyte reduction, and perivascular depletion of microglia in a 6-OHDA toxin model of Parkinson disease. J Neuroinflammation. 2021;18(1):175. https://doi.org/10.1186/s12974-021-02218-8.
Tabitha Green. (2019). Shape-shifting Brain Cells. ASU - Ask A Biologist. Retrieved January 8, 2024 from https://askabiologist.asu.edu/plosable/shape-shifting-brain-cells.
Arba F, Leigh R, Inzitari D, Warach SJ, Luby M, Lees KR. Blood–brain barrier leakage increases with small vessel disease in acute ischemic stroke. Neurol. 2017;89(21):2143–50. https://doi.org/10.1212/WNL.0000000000004677.
Lai Y, Jiang C, Du X, Sang C, Guo X, Bai R, Tang R, Dong J, Ma C. Effect of intensive blood pressure control on the prevention of white matter hyperintensity: systematic review and meta-analysis of randomized trials. J Clin Hypertens (Greenwich). 2020;22(11):1968–73. https://doi.org/10.1111/jch.14030.
Giezendanner S, Fisler MS, Soravia LM, Andreotti J, Walther S, Wiest R, Dierks T, Federspiel A. Microstructure and cerebral blood flow within white matter of the human brain: a TBSS analysis. PLoS ONE. 2016;11(3): e0150657. https://doi.org/10.1371/journal.pone.0150657.
Ritz MF, Grond-Ginsbach C, Kloss M, Tolnay M, Fluri F, Bonati H, Traenka L, Zeis C, Schaeren-Wiemers T, Peters N, Engelter N, Engelter ST, Alexandre Lyrer P. Identification of inflammatory, metabolic, and cell survival pathways contributing to cerebral small vessel disease by postmortem gene expression microarray. Curr Neurovasc Res. 2016;13(1):58–67. https://doi.org/10.2174/1567202612666151027151025.
Murray ME, Vemuri P, Preboske GM, Murphy MC, Schweitzer KJ, Parisi JE, Jack CR Jr, Dickson DW. A quantitative postmortem MRI design sensitive to white matter hyperintensity differences and their relationship with underlying pathology. Neuropathol Exp Neurol. 2012;71(12):1113–22. https://doi.org/10.1097/NEN.0b013e318277387e.
Ritz MF, Fluri F, Engelter ST, Schaeren-Wiemers N, Lyrer PA. Cortical and putamen age-related changes in the microvessel density and astrocyte deficiency in spontaneously hypertensive and stroke-prone spontaneously hypertensive rats. Curr Neurovasc Res. 2009;6(4):279–87. https://doi.org/10.2174/156720209789630311.
Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2010;59(1):228–35. https://doi.org/10.2337/db09-0902.
Prakash R, Johnson M, Fagan SC, Ergul A. Cerebral neovascularization and remodeling patterns in two different models of type 2 diabetes. PLoS ONE. 2013;8(2): e56264. https://doi.org/10.1371/journal.pone.0056264.
Zhang, H., Xu, R., Wang, Z., Contribution of oxidative stress to HIF-1-mediated profibrotic changes during the kidney damage. Oxid Med Cell Longev. 2021, 6114132. https://doi.org/10.1155/2021/6114132
Cao Y, Li Z, Li H, Ni C, Li L, Yang N, Shi C, Zhong Y, Cui D, Guo X. Hypoxia-inducible factor-1α is involved in isoflurane-induced blood-brain barrier disruption in aged rats model of POCD. Behav Brain Res. 2018;339:39–46. https://doi.org/10.1016/j.bbr.2017.09.004.
Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106(6):1977–82. https://doi.org/10.1073/pnas.080869810.
Terashima J, Sampei S, Iidzuka M, Ohsakama A, Tachikawa C, Satoh J, Kudo K, Habano W, Ozawa S. VEGF expression is regulated by HIF-1α and ARNT in 3D KYSE-70, esophageal cancer cell spheroids. Cell Biol Int. 2016;40(11):1187–94. https://doi.org/10.1002/cbin.10656.
Barallobre-Barreiro J, Loeys B, Mayr M, Rienks M, Verstraeten A, Kovacic JC. Extracellular matrix in vascular disease, part 2/4: JACC Focus Seminar. J Am Coll Cardiol. 2020;75(17):2189–203. https://doi.org/10.1016/j.jacc.2020.03.018.
Martinez-Quinones P, McCarthy CG, Watts SW, Klee NS, Komic A, Calmasini FB, Priviero F, Warner A, Chenghao Y, Wenceslau CF. Hypertension induced morphological and physiological changes in cells of the arterial wall. Am J Hypertens. 2018;31(10):1067–78. https://doi.org/10.1093/ajh/hpy083.
Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288(15):10819–29. https://doi.org/10.1074/jbc.M112.442939.
Iglesias-de la Cruz MC, Ziyadeh FN, Isono M, Kouahou M, Han DC, Kalluri R, Mundel P, Chen S. Effects of high glucose and TGF-beta1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62(3):901–13. https://doi.org/10.1046/j.1523-1755.2002.00528.x.
Barrows IR, Ramezani A, Raj DS. Inflammation, immunity, and oxidative stress in hypertension-partners in crime? Adv Chronic Kidney Dis. 2019;26(2):122–30. https://doi.org/10.1053/j.ackd.2019.03.001.
Bruce-Keller AJ, White CL, Gupta S, Knight AG, Pistell PJ, Ingram DK, Morrison CD, Keller JN. NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med. 2010;49(1):22–30. https://doi.org/10.1016/j.freeradbiomed.2010.03.006.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2017;114(12):1752–61. https://doi.org/10.1172/JCI21625.
Pepping JK, Vandanmagsar B, Fernandez-Kim SO, Zhang J, Mynatt RL, Bruce-Keller AJ. Myeloid-specific deletion of NOX2 prevents the metabolic and neurologic consequences of high fat diet. PLoS ONE. 2017;12(8): e0181500. https://doi.org/10.1371/journal.pone.0181500.
Acknowledgements
We would like to acknowledge the technical expertise of the Interdisciplinary Center for Science Research, Organization for Research and Academic Information, and Department of Experimental Animals, Interdisciplinary Center for Science Research, Shimane University. We would also like to thank Yasuko Wada, Abul Kalam Azad, Yuxin Liu, Xinlang Liu, and Pang Bo for their technical support during the experiments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Yuchi Zhang: Conceptualization, Data curation, Methodology, Writing – original draft. Abdullah Md. Sheikh: Conceptualization, Methodology, Writing – review & editing. Shatera Tabassum: Methodology, Supervision, Validation. Kenichi Iwasa: Supervision, Validation. Abu Zaffar Shibly: Methodology, Supervision. Xiaojing Zhou: Validation. Ruochen Wang: Validation. Jubo Bhuiya: Methodology, Validation. Abdullah Fatema Binte: Validation. Shozo Yano: Validation. Yoshihito Aoki: Validation. Atsushi Nagai: Conceptualization, Project administration, Supervision, Writing – review & editing. All authors provided critical feedback and contributed to the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animals were used according to the ARRIVE reporting guidelines (Animal Research: Reporting of In Vivo Experiments), and guidelines of the Institute of Experimental Animals of Shimane University. The experimental protocols and procedures were approved by the Ethical Committee of Shimane University (approval code: IZ2-96).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zhang, Y., Sheikh, A.M., Tabassum, S. et al. Effect of high-fat diet on cerebral pathological changes of cerebral small vessel disease in SHR/SP rats. GeroScience (2024). https://doi.org/10.1007/s11357-024-01074-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01074-7