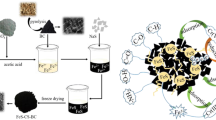
Abstract
Nowadays, clean-up of heavy metals from wastewaters using waste residue carbon-based material has received increasing attention. In this work, a novel Chinese medicine residue carbon-based nano zero-valent iron composite (CM-nZVI) had been successfully prepared using the combined Chinese medicine residue, FeCl3 and green tea extract. Cr(VI) and/ or Cd(II) removal in water by the CM-nZVI were systematacially investigated with a series of batch experiments. The most relevant findings indicated the adsorption efficiecy and capacity of Cr(VI) by CM-nZVI were respecitvely nearly 98% and 26 mg/g under optimized reaction conditions. The negative influences of the cations on the Cr(VI) removal followed the order of Al3+ > Ca2+ > Mg2+ Na+ > K+, but the anions followed the order of HCO3− > PO43− > NO3− > Cl− > SO42−. Humic acid (HA) and ionic strength with high concentrations severely inhibited Cr(VI) removal. The Cr(VI) adsorption on CM-nZVI fitted well by the pseudo-second-order kinetic and Langmuir models. A monolayer endothermic chemisorption occurred on Cr(VI) adsorption over CM-nZVI, and Cr(VI) removal by CM-nZVI primarily involved in the absorption, reduction, precipitation and complexation processes. Both Cr(VI) and Cd(II) removals had been achieved by CM-nZVI at their low concentrations. This CM-nZVI showed a better reusability proprity for Cr(VI) and Cd(II) removal with the regeneration of CM-nZVI through simple pickling. The outcomes of this work show that CM-nZVI could be used an effective material for heavy metals removal from water.
Graphical Abstract











Similar content being viewed by others
Data availability
The data used to support the results of the present investigation are involved in the article.
References
Awual MR, Khraisheh M, Alharthi NH, Luqmanc M, Islam A, Karim MR, Rahman MM, Khaleque MA (2018) Efficient detection and adsorption of cadmium(II) ions using innovative nano-composite materials. Chem Eng J 343:118–127
Bian RJ, Chen D, Liu XY, Cui LQ, Li LQ, Pan GX, Xie D, Zheng JW, Zhang XH, Zheng JF, Chang A (2013) Biochar soil amendment as a solution to prevent Cd-tainted rice from China: results from a cross-site field experiment. Ecol Eng 58:378–383
Chandel M, Thakur M, Sharma A, Pathania D, Kumar A, Singh L (2022) Chlorophyll sensitized (BiO)2CO3/CdWO4/rGO nano-hybrid assembly for solar assisted photo-degradation of chlorzoxazone. Chemosphere 305:135472
Chen SJ, Zhang JS, Zhang HW, Wang XK (2017) Removal of hexavalent chromium from contaminated water by Chinese herb-extraction residues. Water, Air, Soil Pollut 228:145
Chen MX, He FF, Hu DW, Bao CZ, Huang Q (2020) Broadened operating pH range for adsorption/reduction of aqueous Cr(VI) using biochar from directly treated jute (Corchorus capsularis L.) fibers by H3PO4. Chem Eng J 381:122739
Diao ZH, Xu XR, Jiang D, Kong LJ, Sun YX, Hu YX, Hao QW, Chen H (2016) Bentonite-supported nanoscale zero-valent iron/persulfate system for the simultaneous removal of Cr(VI) and phenol from aqueous solutions. Chem Eng J 302:213–222
Diao ZH, Xu XR, Jiang D, Liu JJ, Kong LJ, Li G, Zou LZ, Wu QH (2017) Simultaneous photocatalytic Cr(VI) reduction and ciprofloxacin oxidation over TiO2/Fe0 composite under aerobic conditions: performance, durability, pathway and mechanism. Chem Eng J 305:167–176
Diao ZH, Du JJ, Jiang D, Kong LJ, Huo WY, Liu CM, Wu QH, Xu XR (2018) Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludgederived biochar immobilized nanoscale zero valent iron: coexistence effects and mechanism. Sci Total Environ 642:505–515
Diao ZH, Dong FX, Yan L, Chen ZL, Qian W, Kong LJ, Zhang ZW, Zhang T, Tao XQ, Du JJ, Jiang D, Chu W (2020a) Synergistic oxidation of Bisphenol A in a heterogeneous ultrasound-enhanced sludge biochar catal yst/persulfate process: reactivity and mechanism. J Hazard Mater 384:121385
Diao ZH, Yan L, Dong FX, Qian W, Deng QH, Kong LJ, Yang JW, Lei ZX, Du JJ, Chu W (2020b) Degradation of 2,4-dichlorophenol by a novel iron based system and its synergism with Cd(II) immobilization in a contaminated soil. Chem Eng J 379:122313
Diao ZH, Qian W, Zhang ZW, Jin JC, Chen ZL, Guo PR, Dong FX, Yan L, Kong LJ, Chu W (2020c) Removals of Cr(VI) and Cd(II) by a novel nanoscale zero valent iron/peroxydisulfate process and its Fenton-like oxidation of pesticide atrazine: coexisting effect, products and mechanism. Chem Eng J 397:125382
Diao ZH, Yan L, Dong FX, Chen ZL, Guo PR, Qian W, Zhang WX, Liang JY, Huang ST, Chu W (2021) Ultrasound-assisted catalytic reduction of Cr(VI) by an acid mine drainage based nZVI coupling with FeS2 system from aqueous solutions: performance and mechanism. J Environ Manag 278:111518
Diao ZH, Jin JC, Zou MY, Liu H, Qin JQ, Zhou XH, Qian W, Guo PR, Kong LJ, Chu W (2022) Simultaneous degradation of amoxicillin and norfloxacin by TiO2@ nZVI composites coupling with persulfate: Synergistic effect, products and mechanism. Sep Purif Technol 278:119620
Dong FX, Yan L, Zhou XH, Huang ST, Liang JY, Zhang WX, Guo ZW, Guo PR, Qian W, Kong LJ, Chu W, Diao ZH (2021) Simultaneous adsorption of Cr(VI) and phenol by biochar-based iron oxide composites in water: Performance, kinetics and mechanism. J Hazard Mater 416:125–930
Dong FX, Yan L, Huang ST, Liang JY, Zhang WX, Yao XW, Chen X, Qian W, Guo PR, Kong LJ, Chu W, Diao ZH (2022) Removal of antibiotics sulfadiazine by a biochar based material activated persulfate oxidation system: Performance, products and mechanism. Process Saf Environ 157:411–419
Huang LL, Weng XL, Chen ZL, Megharaj M, Naidu R (2014) Green synthesis of iron nanoparticles by various tea extracts: Comparative study of the reactivity. Spectrochim Acta a 130:295–301
Jiang Q, Jiang SM, Zhang R, Jiang Z, Zhang Y (2022) A stable biochar supported S-nZVI to activate persulfate for effective dichlorination of atrazine. Chem Eng J 431:133937
Kumar A, Sharma K, Thakur M, Pathania D, Sharma A (2022) Fabrication of high visible light active LaFeO3/Cl-g-C3N4/RGO heterojunction for solar assisted photo-degradation of aceclofenac. J Environ Chem Eng 10:108098
Lin H, Han SK, Dong YB, He YH (2017) The surface characteristics of hyperbranched polyamide modified corncob and its adsorption property for Cr(VI). Appl Surf Sci 412:152–159
Liu CM, Diao ZH, Huo WY, Kong LJ, Du JJ (2018) Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: synthesis, reactivity and mechanism. Environ Pollut 239:698–705
Lu Q, Li CL (2021) Comprehensive utilization of Chinese medicine residues for industry and environment protection: Turning waste into treasure. J Clean Prod 279:123856
Mukherjee A, Zimmerman AR (2013) Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 193–194:122–130
Qian W, Liang JY, Zhang WX, Huang ST, Diao ZH (2022) A porous biochar supported nanoscale zero-valent iron material highly efficient for the simultaneous remediation of cadmium and lead contaminated soil. J Environ Sci 13:231–241
Qiu BB, Tao XD, Wang H, Li WK, Ding X, Chu HQ (2021) Biochar as a low-cost adsorbent for aqueous heavy metal removal: a review. J Anal Appl Pyrol 155:105081
Shao PL, Yin H, Li YC, Cai YH, Yan CY, Yuan YB, Dang Z (2023) Remediation of Cu and As contaminated water and soil utilizing biochar supported layered double hydroxide: Mechanisms and soil environment altering. J Environ Sci 126:275–286
Sharma A, Deepak Pathania D, Kumar A (2020) Bio-Polymer Based Tragacanth Gum (TG) Loaded Fe3O4 Nanocomposite for the Sequestration of Tenacious Congo Red Dye from Waste Water. J Mater Sci Technol Res 7:92–100
Wang DF, Yang PQ, Chen CW, Wu QC, Wu ZY, Cai DQ (2011) Simultaneously removal of Cr(VI) and Cd(II) from water using a flower-like primary battery nanosystem. Sci Total Environ 765:142735
Wang T, Lin JJ, Chen ZL, Megharaj M, Naidu R (2014) Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J Clean Prod 83:413–419
Wang AQ, Zheng ZK, Li RQ, Hu D, Lu YR, Luo HX, Yan K (2019) Biomass-derived porous carbon highly efficient for removal of Pb(II) and Cd(II). Green Energy Environ 4:414–423
Wang HG, Lou XX, Hu Q, Sun T (2021) Adsorption of antibiotics from water by using Chinese herbal medicine residues derived biochar: Preparation and properties studies. J Mol Liq 325:114967
Xu YY, Wu YH, Wu XK, Sun YT, Huang ZL, Li H, Wu ZJ, Zhang X, Qin XL, Zhang YR, Deng JQ, Huang J (2022) Effect of physicochemical properties of biochar from different feedstock on remediation of heavy metal contaminated soil in mining area. Surf Interfaces 32:102058
Yan L, Dong FX, Lin X, Zhou XH, Kong LJ, Chu W, Diao ZH (2021) Insights into the removal of Cr(VI) by a biochar-iron composite from aqueous solution: reactivity, kinetics and mechanism. Environ Technol Inno 24:102057
Yan L, Dong FX, Li Y, Guo PR, Kong LJ, Chu W, Diao ZH (2022) Synchronous removal of Cr(VI) and phosphates by a novel crayfish shell biochar-Fe composite from aqueous solution: Reactivity and mechanism. J Environ Chem Eng 10:107396
Yi Y, Wang XY, Zhang YX, Ma J, Ning P (2022) Adsorption properties and mechanism of Cr(VI) by Fe2(SO4)3 modified biochar derived from Egeria najas. Colloid Surface a 645:128938
Yin GC, Song XW, Tao L, Sarkar B, Sarmah AK, Zhang WX, Lin QT, Xiao RB, Liu QJ, Wang HL (2020) Novel Fe-Mn binary oxide-biochar as an adsorbent for removing Cd(II) from aqueous solutions. Chem Eng J 389:124465
Yu ZW, Gao Q, Zhang Y, Wang DD, Nyalala I, Chen KJ (2019) Production of activated carbon from sludge and herb residue of traditional Chinese medicine industry and its application for methylene blue removal. Bio Resources 14:1333–1346
Yuan HR, Lu T, Wang YZ, Huang HY, Chen Y (2014) Influence of pyrolysis temperature and holding time on properties of biochar derived from medicinal herb (radix isatidis) residue and its effect on soil CO2 emission. J Anal Appl Pyrol 110:277–284
Zhang X, Wang H, He L, Lu K, Sarmah A, Li J, Bolan NS, Pei J, Huang H (2013) Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res 20:8472–8483
Zhang YT, Jiao XQ, Liu N, Lv J, Yang YD (2020) Enhanced removal of aqueous Cr(VI) by a green synthesized nanoscale zero-valent iron supported on oak wood biochar. Chemosphere 245:125542
Zhang WX, Chen X, Xiao GS, Liang JY, Kong LJ, Yao XW, Diao ZH (2023) A novel pigeon waste based biochar composite for the removal of heavy metal and organic compound: Performance, products and mechanism. Colloid Surf a 551:131277
Zhou Y, Selvam A, Wong JWC (2018) Chinese medicinal herbal residues as a bulking agent for food waste composting. Bioresour Technol 249(182):229–234
Zhu F, Ma SY, Liu T, Deng XQ (2018) Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J Clean Prod 174:84–190
Zou HW, Hu ED, Yang SY, Gong L, He F (2019) Chromium(VI) removal by mechanochemically sulfidated zerovalent iron and its effect on dechlorination of trichloroethene as a co-contaminant. Sci Total Environ 650:419–426
Funding
This work was supported by the Special project in key areas of Guangdong Province Ordinary Universities (Nos.2020ZDZX1003 and 2021ZDJS007), the Key Realm Research and Development Program of Guangdong Province (No.2020B1111350002), Xingning Meat Pigeon Provincial Agricultural Industrial Park Project (No.D122222G913), the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (No.2019KJ140), the National Natural Science Foundation of China (Nos.U22A20606 and 21407155) and Zhongkai Graduate Science and Technology Innovation Fund Project (No.KJCX2023023).
Author information
Authors and Affiliations
Contributions
Conceptualization, Investigation and Methodology, Fa-Ming Diao; Investigation, Methodology and Software, Man-Li Chen; Investigation and Software, Lin-Yin Tong and Ying-Nan Chen; Conceptualization, Writing-original draft, Writing review &editing and Supervision, Zeng-Hui Diao.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current study does not include any animals.
Consent to participate
Not applicable.
Consent for publication
All the authors agree for the publication, and the present manuscript does not contain data from any person solely.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ioannis A. Katsoyiannis
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diao, FM., Chen, ML., Tong, LY. et al. A green synthesized medicine residue carbon-based iron composite for the removal of chromium (VI) and cadmium (II): Performance, kinetics and mechanism. Environ Sci Pollut Res 30, 84011–84022 (2023). https://doi.org/10.1007/s11356-023-28429-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28429-5