Abstract
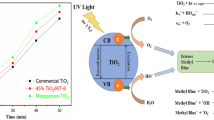
Mesoporous silica (MCM-48) was synthesized and used as a catalyst for supporting the nickel oxide photocatalyst. The loading of nickel oxide on MCM-48 results in a considerable reduction in the bandgap energy to 2.4 eV. MCM-48 was used as a catalyst and back-supporter for the nickel oxide to enhance its photocatalytic properties along with adsorption capacity. Therefore, the adsorption capacity of MCM-48/Ni2O3 was enhanced by 17.5% and 32.2% compared to Ni2O3 and MCM-48, respectively. Furthermore, the percentage of photocatalytic degradation was improved by approximately 68.2% relative to the free-standing Ni2O3. The MCM-48/Ni2O3 proved the chemisorption adsorption mechanism that happens in multilayer form through the heterogeneous surface. This through fixing such Ni2O3 particles over the nanoporous topography to provide more exposed hot adsorption and photocatalytic sites for the incident light photons. Therefore, supporting Ni2O3 catalytic particles onto MCM-48 produces a new category of photocatalytic systems with promising active centers for the efficient degradation of Congo red dye molecules.












Similar content being viewed by others
References
Abudaia JA, Sulyman MO, Elazaby KY, Ben-Ali SM (2013) Adsorption of Pb (II) and cu (II) from aqueous solution onto activated carbon prepared from dates stones. Int J Environ Sci Develop 2:191–195
Akbal F, van Driel BA et al (2015) Photocatalytic degradation of organic dyes in the presence of titanium dioxide under UV and solar light: effect of operational parameters. Environ Prog 24:317–322
Alireza NE, Shahriari E (2014) Photocatalytic decolorization of methyl green using Fe(II)-o-phenanthroline as supported onto zeolite Y. Ind Eng Chem 20:2719–2726
Bagherifam S, Komarneni S, Lakzian A, Fotovat A, Khorasani R, Huang W, Ma J, Hong S, Cannon FS, Wang Y (2014) Highly selective removal of nitrate and perchlorate by organoclay. Appl Clay Sci 95:126–132
Behnajady MA, Modirshahla N, Hamzavi R (2006) Kinetic study on photocatalytic degradation of CI acid yellow 23 by ZnO photocatalyst. J Hazard Mater B 133:226–232
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186:458–465
Chaouch N, Ouahrani MR, Laouini SE (2014) Adsorption of lead (II) from aqueous solutions onto activated carbon prepared from Algerian dates stones of Phoenix dactylifera L (Ghars variety) by H3 PO4 activation. Orient J Chem 30:1317–1322
Chrysicopoulou P, Davazoglou D, Trapalis C, Kordas G (1998) Optical properties of very thin (< 100 nm) sol-gel TiO2 films. Thin Solid Films 323:188–193
Dharmaraja N, Prabuc P, Nagarajand S, Kimb CH, Parkb JH, Kimb HY (2006) Synthesis of nickel oxide nanoparticles using nickel acetate and poly(vinyl acetate) precursor. Mater Sci & Engin: B 128:111–114
Dimitrakopoulou D, Rethemiotaki I, Frontistis Z, Xekoukoulotakis NP, Venieri D, Mantzavinos D (2012) Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J Environ Manag 98:168e174
Duraisamya R, Kiruthigaa PM, Hirpayeb BY, Berekuteb AK (2015) Adsorption of azure B dye on rice husk activated carbon: equilibrium, kinetic and thermodynamic studies. Int J Water Res 5(2):18–28
Fathal ES, Ahmed LM (2015) Optimization of photocatalytic decolourization of methyl green dye using commercial zinc oxide as catalyst. Journal of Kerbala University, 13 No. 1 Scientific
Giles CH, McEwan TH, Nakhawa SN, Smith D (1960) Studies in adsorption: part XI. A system of classification of solution adsorption isotherms and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc:3973–3993
Groisman L, Rav-Acha C, Gerstl Z, Mingelgrin U (2004) Sorption of organic compounds of varying hydrophobicities from water and industrial wastewater by long- and short-chain organoclays. Appl Clay Sci 24:159–166
Gupta VK, Mohan D, Saini VK (2006) Studies on the interaction of some azo dyes (naphthol red-J and direct orange) with nontronite mineral. J Colloid Interface Sci 298:79–86
Hinz C (2001) Description of sorption data with isotherm equations. Geoderma 99:225–243
Huang YH, Huang YF, Chang PS, Chen CY (2008) Comparative study of oxidation of dye-reactive black B by different advanced oxidation processes: Fenton, electro-Fenton and photo-Fenton. J Hazard Mater 154:655–662
Jiang G, Nana T, Jie L, Kun Y, Zhiwei J, Xuecheng C, Xin W, Ewa M, Tao T (2014) Synergistic effect of activated carbon and Ni2O3 in promoting the thermal stability and flame retardancy of polypropylene. Polym Degrad Stab 99:18–26
Khezrianjoo S, Revanasiddappa HD (2013) Photocatalytic degradation of acid yellow 36 using zinc oxide photocatalyst in aqueous media. J Catal 582058:6
Khmissi H, El Sayed AM, Shaban M (2016) Structural, morphological, optical properties and wettability of spin-coated copper oxide; influences of film thickness, Ni, and (La, Ni) co-doping. J Mater Sci 51:5924–5938. https://doi.org/10.1007/s10853-016-9894-7
Ko H, Yang G, Wang M, Zhao X (2014) Isothermal crystallization kinetics and effect of crystallinity on the optical properties of nanosized CeO2 powder. Ceram Int 40:6663–6671
Li H, Mab H, Yang M, Wang B, Shao H, Le W, Yu R, Wang D (2017) Highly controlled synthesis of multi-shelled NiO hollow microspheres for enhanced lithium storage properties. Mater Research Bull 87:224–229
Mohamed F, Abukhadra MR, Shaban M (2018) Removal of safranin dye from water using polypyrrole nanofiber/Zn-Fe layered double hydroxide nanocomposite (Ppy NF/Zn-Fe LDH) of enhanced adsorption and photocatalytic properties. Sci Total Environ 640–641:352–363
Narges A, Alireza NE (2015) Modification of clinoptilolite nano-particles with iron oxide: increased composite catalytic activity for photodegradation of cotrimoxazole in aqueous suspension. Mater Sci Semicond Process 31:684–692
Perera SD, Mariano RG, Vu K, Nour N, Seitz O, Chabal Y, Balkus KJ (2012) Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal 2:949–995
Rahman A, Urabe T, Kishimoto N (2013) Color removal of reactive procion dyes by clay adsorbents. Proc Environ Sci 17:270–278
Rakshitha R, Yashas SR (2017) Removal of nitrate from water by chitosan, clay and activated carbon as adsorbents: a mini review. Int J Eng Res Appl 7(10):46–51
Ramachandra TV, Varghese SK (2003) Exploring possibilities of achieving sustainability in solid waste management. Environ Health 45(4):255–264
Razieh N, Ali RM, Zahra H, Tahereh A (2005) Pd-functionalized MCM-41 nanoporous silica as an efficient and reusable catalyst for promoting organic reactions. RSC Adv 5:16029–16035
Reza KM, Kurny ASW, Gulshan F (2015) Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci. https://doi.org/10.1007/s13201-015-0367-y
Sayan D, Swarupananda BB, Mahua GC, Raj SB, Suman H, Chandan KG (2015) Synthesis of pure nickel(III) oxide nanoparticles at room temperature for Cr(VI) ion removal. RSC Adv 5:54717–54726
Shaban M, Abukhadra MR (2017) Geochemical evaluation and environmental application of Yemeni natural zeolite as sorbent for Cd2+ from solution: kinetic modeling, equilibrium studies, and statistical optimization. Environ Earth Sci 76:2–16
Shaban M, Abukhadra MR, Hamd A, Amin RR, Khalek AA (2017) Photocatalytic removal of Congo red dye using MCM-48/Ni2O3 composite synthesized based on silica gel extracted from rice husk ash; fabrication and application. J Environ Manag 204:198–199
Sharma J, Janveja B (2008) A study on the removal of Congo red dye from the effluents of textile industry using rice husk carbon activated by steam. Rasayan J Chem 1:936–942
Shengfang L, Min T (2015) Removal of cationic dyes from aqueous solutions by a low-cost biosorbent longan shell. Desalination & Water Treatment:1–7. https://doi.org/10.1080/19443994.2014.1001444
Siddique R, Iqbal Khan M (2011) Supplementary cementing materials, engineering materials, Springer-Verlag Berlin Heidelberg, DOI: https://doi.org/10.1007/978-3-642-17866-5_2
Silica Fume Association (2005( Silica fume manual. 38860 Sierra Lane, Lovettsville, VA 20180, USA
Sun JH, Sun SP, Sun JY, Sun RX, Qiao LP, Guo HQ, Fan MH (2007) Degradation of azo dye acid black 1 using low concentration iron of Fenton process facilitated by ultrasonic irradiation. J Ult Sonochem 14:761–766
Sun D, Zhang Z, Wang M, Wu Y (2013) Adsorption of reactive dyes on activated carbon developed from Enteromorpha prolifera. American J Anal Chem 4:17–26
Tarigh GD, Shemirani F, Maz'hari NS (2015) Fabrication of a reusable magnetic multi-walled carbon nanotube–TiO2 nanocomposite by electrostatic adsorption: enhanced photodegradation of malachite green. RSC Adv 5:35070–35079
Temkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:217–222
Wang N, Li J, Zhu L, Dong Y, Tang H (2008) Highly photocatalytic activity of metallic hydroxide/titanium dioxide nanoparticles prepared via a modified wet precipitation process. J Photochem Photobiol A 198:282–287
Williamson GK, Smallman RE (1956) Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray Debye-Scherrer spectrum. Philos Mag 1:34–46
Yongde X, Robert M (2003) Facile and high yield synthesis of mesostructured MCM-48 silica crystals. J Mater Chem 13:657–659
Zhang B, Shi R, Zhang Y, Pan C (2013) CNTs/TiO2composites and its electrochemical properties after UV light irradiation. Prog Nat Sci Mater Int 23(2):164–169
Zhen W, Xinbo C, Hongjun D, Lu Y, Huixiang S (2012) One-step synthesis of highly active Ti-containing Cr-modified MCM-48 mesoporous material and the photocatalytic performance for decomposition of H2S under visible light. Appl Surface Sci 258:8258–8263
Zohra N, Riaz KS, Naseem S, Zia R (2016) Synthesis and characterization of Ni2O3 thin films. The World Congress on Advances in Civil, Environmental, and Materials Research, Korea
Zouzelka R, Kusumawati Y, Remzova M, Rathousky J, Pauporté T (2016) Photocatalytic activity of porous multiwalled carbon nanotube-TiO2 composite layers for pollutant degradation. J Hazard Mater 317:52–59
Acknowledgments
The Egyptian Academy partially supported this work for scientific research and technology (ASRT/1515/2017).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Sami Rtimi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaban, M., Hamd, A., Amin, R.R. et al. Preparation and characterization of MCM-48/nickel oxide composite as an efficient and reusable catalyst for the assessment of photocatalytic activity. Environ Sci Pollut Res 27, 32670–32682 (2020). https://doi.org/10.1007/s11356-020-09431-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09431-7