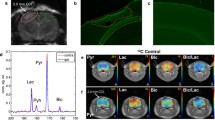
Abstract
Purpose
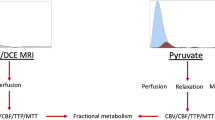
This study examined the feasibility of using two novel agents, hyperpolarized [13C]t-butanol and [13C,15N2]urea, for assessing in vivo perfusion of the intact spinal cord in rodents. Due to their distinct permeabilities to blood brain barrier (BBB), we hypothesized that [13C]t-butanol and [13C,15N2]urea exhibit unique 13C signal characteristics in the spinal cord.
Procedures
Dynamic 13C t-butanol MRI data were acquired from healthy Long-Evans rats using a symmetric, ramp-sampled, partial-Fourier 13C echo-planar imaging sequence after the injection of hyperpolarized [13C]t-butanol solution. In subsequent scans, dynamic 13C urea MRI data were acquired after the injection of hyperpolarized [13C,15N2]urea. The SNRs of t-butanol and urea were calculated for regions corresponding to spine, supratentorial brain, and blood vessels and plotted over time. Mean peak SNR and AUC were calculated from the dynamic plots for each region and compared between t-butanol and urea.
Results
In spine and supratentorial brain, the mean peak SNR and AUC of t-butanol were significantly higher than those of urea (p < 0.05). In contrast, urea was predominantly contained within vasculature and exhibited significantly higher levels of mean peak SNR and AUC compared to t-butanol in blood vessels (p < 0.05).
Conclusion
This study has demonstrated the feasibility of using hyperpolarized [13C]t-butanol and [13C,15N2]urea for assessing in vivo perfusion in cervical spinal cord. Due to differences in blood–brain barrier permeability, t-butanol rapidly crossed the blood–brain barrier and diffused into spine and brain tissue, while urea predominantly remained in vasculature. The results from this study suggest that this technique may provide unique non-invasive imaging tracers that are able to directly monitor hemodynamic processes in the normal and injured spinal cord.


Similar content being viewed by others
References
Mautes AE, Weinzierl MR, Donovan F, Noble LJ (2000) Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther 80:673–687
Stroman PW, Wheeler-Kingshott C, Bacon M et al (2014) The current state-of-the-art of spinal cord imaging: methods. Neuroimage 84:1070–1081
Vargas MI, Delattre BMA, Boto J et al (2018) Advanced magnetic resonance imaging (MRI) techniques of the spine and spinal cord in children and adults. Insights Imaging 9:549–557
Vargas MI, Delavelle J, Kohler R, Becker CD, Lovblad K (2009) Brain and spine MRI artifacts at 3Tesla. J Neuroradiol 36:74–81
Wang ZJ, Ohliger MA, Larson PEZ et al (2019) Hyperpolarized (13)C MRI: state of the art and future directions. Radiology 291:273–284
Gallagher FA, Woitek R, McLean MA et al (2020) Imaging breast cancer using hyperpolarized carbon-13 MRI. Proc Natl Acad Sci USA 117:2092–2098
Chen HY, Aggarwal R, Bok RA et al (2020) Hyperpolarized (13)C-pyruvate MRI detects real-time metabolic flux in prostate cancer metastases to bone and liver: a clinical feasibility study. Prostate Cancer Prostatic Dis 23:269–276
Autry AW, Gordon JW, Chen HY et al (2020) Characterization of serial hyperpolarized (13)C metabolic imaging in patients with glioma. NeuroImage Clin 27:102323
Park I, Larson PEZ, Gordon JW et al (2018) Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn Reson Med 80:864–873
Miloushev VZ, Granlund KL, Boltyanskiy R et al (2018) metabolic imaging of the human brain with hyperpolarized (13)C pyruvate demonstrates (13)C lactate production in brain tumor patients. Can Res 78:3755–3760
Cunningham CH, Lau JY, Chen AP et al (2016) Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res 119:1177–1182
Park I, von Morze C, Lupo JM et al (2017) Investigating tumor perfusion by hyperpolarized (13) C MRI with comparison to conventional gadolinium contrast-enhanced MRI and pathology in orthotopic human GBM xenografts. Magn Reson Med 77:841–847
von Morze C, Bok RA, Reed GD, Ardenkjaer-Larsen JH, Kurhanewicz J, Vigneron DB (2014) Simultaneous multiagent hyperpolarized (13)C perfusion imaging. Magn Reson Med 72:1599–1609
McGregor D (2010) Tertiary-butanol: a toxicological review. Crit Rev Toxicol 40:697–727
National Center for Biotechnology Information. PubChem Compound Summary for CID 6386, Tert-Butanol. https://pubchem.ncbi.nlm.nih.gov/compound/Tert-Butanol. Accessed 3 Sept 2021
Svensson J, Månsson S, Johansson E, Petersson JS, Olsson LE (2003) Hyperpolarized 13C MR angiography using trueFISP. Magn Reson Med 50:256–262
Levin VA (1980) Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J Med Chem 23:682–684
Gordon JW, Vigneron DB, Larson PE (2017) Development of a symmetric echo planar imaging framework for clinical translation of rapid dynamic hyperpolarized (13) C imaging. Magn Reson Med 77:826–832
Gordon JW, Hansen RB, Shin PJ, Feng Y, Vigneron DB, Larson PEZ (2018) 3D hyperpolarized C-13 EPI with calibrationless parallel imaging. J Magn Reson 289:92–99
Fuetterer M, Busch J, Peereboom SM et al (2017) Hyperpolarized (13)C urea myocardial first-pass perfusion imaging using velocity-selective excitation. J Cardiovasc Magn Res 19:46
Lau AZ, Miller JJ, Robson MD, Tyler DJ (2016) Cardiac perfusion imaging using hyperpolarized 13c urea using flow sensitizing gradients. Magn Reson Med 75:1474–1483
von Morze C, Larson PE, Hu S et al (2011) Imaging of blood flow using hyperpolarized [(13)C]urea in preclinical cancer models. J Magn Reson Imaging 33:692–697
Chiavazza E, Kubala E, Gringeri CV et al (2013) Earth’s magnetic field enabled scalar coupling relaxation of 13C nuclei bound to fast-relaxing quadrupolar 14N in amide groups. J Magn Reson 227:35–38
Laustsen C, StokholmNørlinger T, Christoffer Hansen D et al (2016) Hyperpolarized 13C urea relaxation mechanism reveals renal changes in diabetic nephropathy. Magn Reson Med 75:515–518
Reed GD, von Morze C, Bok R et al (2014) High resolution (13)C MRI with hyperpolarized urea: in vivo T(2) mapping and (15)N labeling effects. IEEE Trans Med Imaging 33:362–371
Milshteyn E, Reed GD, Gordon JW et al (2020) Simultaneous T(1) and T(2) mapping of hyperpolarized (13)C compounds using the bSSFP sequence. J Magn Reson 312:106691
Grant AK, Vinogradov E, Wang X, Lenkinski RE, Alsop DC (2011) Perfusion imaging with a freely diffusible hyperpolarized contrast agent. Magn Reson Med 66:746–755
Maliborski A, Zukowski P, Nowicki G, Bogusławska R (2011) Contrast-induced nephropathy–a review of current literature and guidelines. Med Sci Mon 17:Ra199-204
Gordon JW, Autry AW, Tang S et al (2020) A variable resolution approach for improved acquisition of hyperpolarized (13) C metabolic MRI. Magn Reson Med 84:2943–2952
Ma J, Park JM (2020) Super-resolution hyperpolarized (13)C imaging of human brain using patch-based algorithm. Tomography 6:343–355
Yang S, Lee J, Joe E et al (2016) Metabolite-selective hyperpolarized (13)C imaging using extended chemical shift displacement at 9.4T. Magn Reson Imaging 34:535–540
Reed GD, von Morze C, Addy NO et al (2016) High spatiotemporal resolution hyperpolarized 13C angiography. J Cardiovasc Magn Reson 18:Q30
Funding
This study was supported by the Ministry of Education, Republic of Korea (2019R1I1A3A01059201), and the Central Medical Service (CMS) Research Fund, Republic of Korea.
Author information
Authors and Affiliations
Contributions
S.J.L., J.G., and I.P. designed the study, collected, and analyzed data. S.J.L. and I.P. wrote the manuscript. J.F.T. and J.G. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, S.J., Park, I., Talbott, J.F. et al. Investigating the Feasibility of In Vivo Perfusion Imaging Methods for Spinal Cord Using Hyperpolarized [13C]t-Butanol and [13C,15N2]Urea. Mol Imaging Biol 24, 371–376 (2022). https://doi.org/10.1007/s11307-021-01682-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01682-1