Abstract
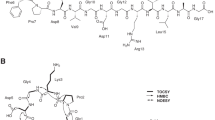
The thermophilic bacterium Thermosporothrix hazakensis belongs to a class of Ktedonobacteria in the phylum Chloroflexota. Lanthipeptides are a naturally occurring peptide group that contains antibacterial compounds such as nisin. To find a new lanthipeptide that is a possible candidate for an antibacterial reagent, we performed genome-mining of T. hazakensis and heterologous expression experiments. Based on genome-mining, the presence of a total of ten putative biosynthetic gene clusters for class I and class II lanthipeptides was indicated from the genome sequence of T. hazakensis. New lanthipeptides named hazakensins A and B were produced by heterologous expression of a class I lanthipeptide biosynthetic gene cluster in the expression host Escherichia coli. Co-expression of the biosynthetic gene cluster with tRNA-Glu and glutamyl-tRNA synthetase coding genes derived from T. hazakensis increased the production yield of both lanthipeptides by about 4–6 times. The chemical structures of hazakensins A and B including the bridging pattern of lanthionine/methyllanthionine rings were determined by NMR and MS experiments. Since production of hazakensins A and B was not observed in the native strain T. hazakensis, heterologous production was an effective method to obtain the lanthipeptides derived from the biosynthetic gene cluster. This is the first report of heterologous production of class I lanthipeptides originating from the filamentous green non-sulfur bacteria, to the best of our knowledge. The success of heterologous production of hazakensins may lead to the discovery and development of new lanthipeptides derived from the origins of bacteria in the phylum Chloroflexota.




Similar content being viewed by others
Data availability
The original data in the study are included in the article/Supplementary Material. The Supplementary Material for this article can be found online. The corresponding author can respond to further inquiries.
References
Anumudu C, Hart A, Miri T, Onyeaka H (2021) Recent advances in the application of the antimicrobial peptide nisin in the inactivation of spore-forming bacteria in foods. Molecules 26:5552. https://doi.org/10.3390/molecules26185552
Baindara P, Nayudu N, Korpole S (2020) Whole genome mining reveals a diverse repertoire of lanthionine synthetases and lanthipeptides among the genus Paenibacillus. J Appl Microbiol 128:473–490. https://doi.org/10.1111/jam.14495
Bothwell IR, Caetano T, Sarksian R, Mendo S, van der Donk WA (2021) Structural analysis of class I lanthipeptides from Pedobacter lusitanus NL19 reveals an unusual ring pattern. ACS Chem Biol 16:1019–1029. https://doi.org/10.1021/acschembio.1c00106
Castiglione F, Lazzarini A, Carrano L, Corti E, Ciciliato I, Gastaldo L, Candiani P, Losi D, Marinelli F, Selva E, Parenti F (2008) Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens. Chem Biol 15:22–31. https://doi.org/10.1016/j.chembiol.2007.11.009
Cavaletti L, Monciardini P, Bamonte R, Schumann P, Rohde M, Sosio M, Donadio S (2006) New lineage of filamentous, spore-forming, gram-positive bacteria from soil. Appl Environ Microbiol 72:4360–4369. https://doi.org/10.1128/AEM.00132-06
Chatterjee C, Paul M, Xie L, van der Donk WA (2005) Biosynthesis and mode of action of lantibiotics. Chem Rev 105:633–684. https://doi.org/10.1021/cr030105v
Cheigh CI, Pyun YR (2005) Nisin biosynthesis and its properties. Biotechnol Lett 27:1641–1648. https://doi.org/10.1007/s10529-005-2721-x
Field D, Cotter PD, Ross RP, Hill C (2015) Bioengineering of the model lantibiotic nisin. Bioengineered 6:187–192. https://doi.org/10.1080/21655979.2015.1049781
Foulston LC, Bibb MJ (2010) Microbisporicin gene cluster reveals unusual features of lantibiotic biosynthesis in actinomycetes. Proc Natl Acad Sci USA 107:13461–13466. https://doi.org/10.1073/pnas.1008285107
Garg N, Tang W, Goto Y, Nair SK, van der Donk WA (2012) Lantibiotics from Geobacillus thermodenitrificans. Proc Natl Acad Sci USA 109:5241–5246. https://doi.org/10.1073/pnas.1116815109
Garg N, Salazar-Ocampo LM, van der Donk WA (2013) In vitro activity of the nisin dehydratase NisB. Proc Natl Acad Sci USA 110:7258–7263. https://doi.org/10.1073/pnas.1222488110
Garrity GM, Holt JG, Castenholz RW, Pierson BK, Keppen OI, Gorlenko VM (2001) Phylum BVI. Chloroflexi phy. nov. In: Boone DR, Castenholz RW, Garrity GM (eds) Bergey’s manual of systematic bacteriology: volume one: the archaea and the deeply branching and phototrophic bacteria. Springer, New York, pp 427–446
Igarashi Y, Yamamoto K, Ueno C, Yamada N, Saito K, Takahashi K, Enomoto M, Kuwahara S, Oikawa T, Tashiro E, Imoto M, Xiaohanyao Y, Zhou T, Harunari E, Oku N (2019) Ktedonoketone and 2’-oxosattabacin, benzenoid metabolites from a thermophilic bacterium Thermosporothrix hazakensis in the phylum Chloroflexi. J Antibiot (Tokyo) 72:653–660. https://doi.org/10.1038/s41429-019-0195-7
Jeanne Dit Fouque K, Hegemann JD, Santos-Fernandez M, Le TT, Gomez-Hernandez M, van der Donk WA, Fernandez-Lima F (2021) Exploring structural signatures of the lanthipeptide prochlorosin 2.8 using tandem mass spectrometry and trapped ion mobility-mass spectrometry. Anal Bioanal Chem 413:4815–4824 https
Kaweewan I, Hemmi H, Komaki H, Kodani S (2020) Isolation and structure determination of a new antibacterial peptide pentaminomycin C from Streptomyces cacaoi subsp. cacaoi. J Antibiot (Tokyo) 73:224–229. https://doi.org/10.1038/s41429-019-0272-y
Kaweewan I, Nakagawa H, Kodani S (2021) Heterologous expression of a cryptic gene cluster from Marinomonas fungiae affords a novel tricyclic peptide marinomonasin. Appl Microbiol Biotechnol 105:7241–7250. https://doi.org/10.1007/s00253-021-11545-y
Kodani S, Hemmi H, Miyake Y, Kaweewan I, Nakagawa H (2018) Heterologous production of a new lasso peptide brevunsin in Sphingomonas subterranea. J Ind Microbiol Biotechnol 45:983–992. https://doi.org/10.1007/s10295-018-2077-6
Lagedroste M, Smits SHJ, Schmitt L (2021) Importance of the leader peptide sequence on the lanthipeptide secretion level. FEBS J 288:4348–4363. https://doi.org/10.1111/febs.15724
Montalban-Lopez M, Scott TA, Ramesh S, Rahman IR, van Heel AJ, Viel JH, Bandarian V, Dittmann E, Genilloud O, Goto Y, Grande Burgos MJ, Hill C, Kim S, Koehnke J, Latham JA, Link AJ, Martinez B, Nair SK, Nicolet Y, Rebuffat S, Sahl HG, Sareen D, Schmidt EW, Schmitt L, Severinov K, Sussmuth RD, Truman AW, Wang H, Weng JK, van Wezel GP, Zhang Q, Zhong J, Piel J, Mitchell DA, Kuipers OP, van der Donk WA (2021) New developments in RiPP discovery, enzymology and engineering. Nat Prod Rep 38:130–239. https://doi.org/10.1039/d0np00027b
Mukherjee S, van der Donk WA (2014) Mechanistic studies on the substrate-tolerant lanthipeptide synthetase ProcM. J Am Chem Soc 136:10450–10459. https://doi.org/10.1021/ja504692v
Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, Nair SK (2015) Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature 517:509–512. https://doi.org/10.1038/nature13888
Ortega MA, Hao Y, Walker MC, Donadio S, Sosio M, Nair SK, van der Donk WA (2016) Structure and tRNA specificity of MibB, a lantibiotic dehydratase from Actinobacteria involved in NAI-107 biosynthesis. Cell Chem Biol 23:370–380. https://doi.org/10.1016/j.chembiol.2015.11.017
Park JS, Kagaya N, Hashimoto J, Izumikawa M, Yabe S, Shin-Ya K, Nishiyama M, Kuzuyama T (2014) Identification and biosynthesis of new acyloins from the thermophilic bacterium Thermosporothrix hazakensis SK20-1(T). ChemBioChem 15:527–532. https://doi.org/10.1002/cbic.201300690
Park JS, Yabe S, Shin-ya K, Nishiyama M, Kuzuyama T (2015) New 2-(1’H-indole-3’-carbonyl)-thiazoles derived from the thermophilic bacterium Thermosporothrix hazakensis SK20-1(T). J Antibiot (Tokyo) 68:60–62. https://doi.org/10.1038/ja.2014.93
Plat A, Kluskens LD, Kuipers A, Rink R, Moll GN (2011) Requirements of the engineered leader peptide of nisin for inducing modification, export, and cleavage. Appl Environ Microbiol 77:604–611. https://doi.org/10.1128/AEM.01503-10
Plat A, Kuipers A, Crabb J, Rink R, Moll GN (2017) Mutagenesis of nisin’s leader peptide proline strongly modulates export of precursor nisin. Antonie Van Leeuwenhoek 110:321–330. https://doi.org/10.1007/s10482-016-0802-6
Repka LM, Chekan JR, Nair SK, van der Donk WA (2017) Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem Rev 117:5457–5520. https://doi.org/10.1021/acs.chemrev.6b00591
Shenkarev ZO, Finkina EI, Nurmukhamedova EK, Balandin SV, Mineev KS, Nadezhdin KD, Yakimenko ZA, Tagaev AA, Temirov YV, Arseniev AS, Ovchinnikova TV (2010) Isolation, structure elucidation, and synergistic antibacterial activity of a novel two-component lantibiotic lichenicidin from Bacillus licheniformis VK21. Biochemistry 49:6462–6472. https://doi.org/10.1021/bi100871b
Singh M, Chaudhary S, Sareen D (2020) Roseocin, a novel two-component lantibiotic from an actinomycete. Mol Microbiol 113:326–337. https://doi.org/10.1111/mmi.14419
Thetsana C, Ijichi S, Kaweewan I, Nakagawa H, Kodani S (2022) Heterologous expression of a cryptic gene cluster from a marine proteobacterium Thalassomonas actiniarum affords new lanthipeptides thalassomonasins A and B. J Appl Microbiol: in print. https://doi.org/10.1111/jam.15491
Tietz JI, Schwalen CJ, Patel PS, Maxson T, Blair PM, Tai HC, Zakai UI, Mitchell DA (2017) A new genome-mining tool redefines the lasso peptide biosynthetic landscape. Nat Chem Biol 13:470–478. https://doi.org/10.1038/nchembio.2319
Unno K, Kaweewan I, Nakagawa H, Kodani S (2020) Heterologous expression of a cryptic gene cluster from Grimontia marina affords a novel tricyclic peptide grimoviridin. Appl Microbiol Biotechnol 104:5293–5302. https://doi.org/10.1007/s00253-020-10605-z
van der Donk WA, Nair SK (2014) Structure and mechanism of lanthipeptide biosynthetic enzymes. Curr Opin Struct Biol 29:58–66. https://doi.org/10.1016/j.sbi.2014.09.006
Vikeli E, Widdick DA, Batey SFD, Heine D, Holmes NA, Bibb MJ, Martins DJ, Pierce NE, Hutchings MI, Wilkinson B (2020) In situ activation and heterologous production of a cryptic lantibiotic from an african plant ant-derived Saccharopolyspora species. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01876-19
Walker MC, Eslami SM, Hetrick KJ, Ackenhusen SE, Mitchell DA, van der Donk WA (2020) Precursor peptide-targeted mining of more than one hundred thousand genomes expands the lanthipeptide natural product family. BMC Genomics 21:387. https://doi.org/10.1186/s12864-020-06785-7
Xu B, Aitken EJ, Baker BP, Turner CA, Harvey JE, Stott MB, Power JF, Harris PWR, Keyzers RA, Brimble MA (2018) Genome mining, isolation, chemical synthesis and biological evaluation of a novel lanthipeptide, tikitericin, from the extremophilic microorganism Thermogemmatispora strain T81. Chem Sci 9:7311–7317. https://doi.org/10.1039/c8sc02170h
Yabe S, Aiba Y, Sakai Y, Hazaka M, Yokota A (2010) Thermosporothrix hazakensis gen. nov., sp. nov., isolated from compost, description of Thermosporotrichaceae fam. nov. within the class Ktedonobacteria Cavaletti 2007 and emended description of the class Ktedonobacteria. Int J Syst Evol Microbiol 60:1794–1801. https://doi.org/10.1099/ijs.0.018069-0
Yu Y, Zhang Q, van der Donk WA (2013) Insights into the evolution of lanthipeptide biosynthesis. Protein Sci 22:1478–1489. https://doi.org/10.1002/pro.2358
Yu Y, Mukherjee S, van der Donk WA (2015) Product formation by the promiscuous lanthipeptide synthetase ProcM is under kinetic control. J Am Chem Soc 137:5140–5148. https://doi.org/10.1021/jacs.5b01409
Zhang Q, Yu Y, Velasquez JE, van der Donk WA (2012) Evolution of lanthipeptide synthetases. Proc Natl Acad Sci USA 109:18361–18366. https://doi.org/10.1073/pnas.1210393109
Zheng Y, Saitou A, Wang CM, Toyoda A, Minakuchi Y, Sekiguchi Y, Ueda K, Takano H, Sakai Y, Abe K, Yokota A, Yabe S (2019) Genome features and secondary metabolites biosynthetic potential of the class Ktedonobacteria. Front Microbiol 10:893. https://doi.org/10.3389/fmicb.2019.00893
Acknowledgements
We thank Ms. Tomoko Sato for the measurement of MS data.
Funding
This study was supported by Grants-in-Aid for Scientific Research: JSPS KAKENHI Grant Number 20K05848 and 21F21095, Institute for Fermentation, OSAKA (IFO): Grant Number G-2021-3-010, and Kobayashi Foundation: Grant Number 196. I. K. was supported by JSPS Postdoctoral Fellowships for Research in Japan (Standard Program).
Author information
Authors and Affiliations
Contributions
IK carried out heterologous production and structure determination of peptides. SI performed molecular cloning. HN performed analysis using NMR and ESI-MS. IK and SK conceived the study and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding this research.
Ethical approval
This study does not contain any experiments with human participants or animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaweewan, I., Ijichi, S., Nakagawa, H. et al. Heterologous production of new lanthipeptides hazakensins A and B using a cryptic gene cluster of the thermophilic bacterium Thermosporothrix hazakensis. World J Microbiol Biotechnol 39, 30 (2023). https://doi.org/10.1007/s11274-022-03463-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03463-6