Abstract
In this work, CoAl-layered double hydroxide (CoAl-LDH) was synthesized by a facile one-step process and utilized as an adsorbent for the removal of thiocyanate (SCN−) from environmental water. The characterization results revealed that CoAl-LDH presents a homogeneous nanosized plate with intercalation of NO3− in the interlayer space. The main factors affecting the removal efficiencies were investigated, and results revealed that CoAl-LDH possessed high removal efficiencies for SCN− and was suitable for a wide range of pH and ambient temperature conditions. Furthermore, the results of the mechanism analysis revealed that the mechanism of adsorption of SCN− by CoAl-LDH mainly includes interlayer ion exchange, electrostatic interactions, and surface ligand exchange. Model fitting of the kinetic data showed that SCN− sorption on CoAl-LDH followed the pseudo-second-order model and the removal rate of SCN− could reach 91.4% with 10 min contact time. Freundlich adsorption isotherm model could describe the adsorption process most accurately, and the maximum adsorption values of SCN− were 187 mg/g at 25℃ and pH 6.0. Meanwhile, the spent CoAl-LDH could be regenerated in Fe(NO3)3 solution and was reused up to four cycles. The overall results demonstrate that CoAl-LDH had a great application potential in the removal of SCN− from aqueous solution.
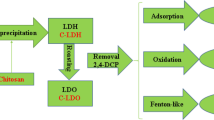
Graphical Abstract











Similar content being viewed by others
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
APHA, A. P. H. A. (2012). Standard methods for the examination of water and wastewater (vol. 22, pp. 1–168). Washington, DC, USA.
Blanchard, G., Maunaye, M., & Martin, G. (1984). Removal of heavy metals from waters by means of natural zeolites. Water Research, 18, 1501–1507.
Budaev, S. L., Batoeva, A. A., & Tsybikova, B. A. (2015). Degradation of thiocyanate in aqueous solution by persulfate activated ferric ion. Minerals Engineering, 81, 88–95.
Chakraborty, S., Sengupta, I., Sarkar, I., Pal, S. K., & Chakraborty, S. (2019). Effect of surfactant on thermo-physical properties and spray cooling heat transfer performance of Cu-Zn-Al LDH nanofluid. Applied Clay Science, 168, 43–55.
Chetal, M., Talwar, D., Singh, R., Arora, S., Bhardwaj, V., Sahoo, S. C. H., Kumar, R., & Sharma, R. (2021). Triethylenetetramine complexes of cobalt(III) having anion binding sites: Synthesis, characterisation, crystal structure, anti-bacterial and anti-cancer properties of [Co(trien)(NO2)2]2Cr2O7 and [Co(trien)(NO2)2]SCN. Journal of Chemical Sciences, 133, 1–12.
Cho, Y., Cattrall, R. W., & Kolev, S. D. (2018). A novel polymer inclusion membrane based method for continuous clean-up of thiocyanate from gold mine tailings water. Journal of Hazardous Materials, 341, 297–303.
Collado, S., Laca, A., & Diaz, M. (2010). Catalytic wet oxidation of thiocyanate with homogeneous copper(II) sulphate catalyst. Journal of Hazardous Materials, 177, 183–189.
Feng, Y. X., Li, C.-Z., Tian, P., & Yu, X.-Z. (2022). Implications of the fate of hydrogen sulfide derived from assimilation of thiocyanate in rice plants. Chemosphere, 306, 135500.
Freundlich, H. (1907). Ueber die adsorption in loesungen. Zeitschrift Für Physikalische Chemie, 57, 385–470.
Gidado, S. M., & Akanyeti, İ. (2020). Comparison of remazol brilliant blue reactive adsorption on pristine and calcined ZnAl, MgAl, ZnMgAl layered double hydroxides. Water, Air, & Soil Pollution, 231, 146.
Gil-Gavilán, D. G., Cosano, D., Castillo-Rodríguez, M., de Miguel, G., Esquivel, D., Jiménez-Sanchidrián, C., Ruiz, J. R., & Romero-Salguero, F. J. (2023). Composites of Co-Al hydrotalcites and carbon nanomaterials for photocatalytic H2 production. Applied Clay Science, 238, 106924.
Gonzalez-Merchan, C., Genty, T., Bussiere, B., Potvin, R., Paquin, M., Benhammadi, M., & Neculita, C. M. (2016). Ferrates performance in thiocyanates and ammonia degradation in gold mine effluents. Minerals Engineering, 95, 124–130.
Gould, W. D., King, M., Mohapatra, B. R., Cameron, R. A., Kapoor, A., & Koren, D. W. (2012). A critical review on destruction of thiocyanate in mining effluents. Minerals Engineering, 34, 38–47.
Hall, K. R., Eagleton, L. C., Acrivos, A., & Vermeulen, T. (1966). Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Industrial & Engineering Chemistry Fundamentals, 5, 212–223.
Hong, D., Zhang, J., Rehman, A. U., Gong, L., Zhou, J., Kan, K., Li, L., & Shi, K. (2016). One-step synthesis of hierarchical Ni-Fe-Al layered double hydroxide with excellent sensing properties for NOx at room temperature. Rsc Advances, 6, 103192–103198.
Huang, P., Liu, J., Wei, F., Zhu, Y., Wang, X., Cao, C., & Song, W. (2017). Size-selective adsorption of anionic dyes induced by the layer space in layered double hydroxide hollow microspheres. Materials Chemistry Frontiers, 1, 1550–1555.
Jawale, R. H., & Gogate, P. R. (2019). Novel approaches based on hydrodynamic cavitation for treatment of wastewater containing potassium thiocyanate. Ultrasonics Sonochemistry, 52, 214–223.
Lagergren, S. (1898). About the theory of so-called adsorption of soluble substances, Kungliga Svenska Vetenskapsakademiens. Handlingar, 24, 1–39.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40, 1361–1403.
Lazaridis, N. K. (2003). Sorption removal of anions and cations in single batch systems by uncalcined and calcined Mg-Al-CO3 hydrotalcite. Water Air and Soil Pollution, 146, 127–139.
Lee, S., Lingamdinne, L. P., Yang, J.-K., Koduru, J. R., Chang, Y.-Y., & Naushad, M. (2022). Biopolymer mixture-entrapped modified graphene oxide for sustainable treatment of heavy metal contaminated real surface water. Journal of Water Process Engineering, 46, 102631.
Li, Y., Gao, B., Wu, T., Chen, W., Li, X., & Wang, B. (2008). Adsorption kinetics for removal of thiocyanate from aqueous solution by calcined hydrotalcite. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 325, 38–43.
Li, L., Yue, F., Li, Y., Yang, A., Li, J., Lv, Y., & Zhong, X. (2020). Degradation pathway and microbial mechanism of high-concentration thiocyanate in gold mine tailings wastewater. Rsc Advances, 10, 25679–25684.
Lin, Y.-J., Yu, X.-Z., Li, Y.-H., & Yang, L. (2020). Inhibition of the mitochondrial respiratory components (Complex I and Complex III) as stimuli to induce oxidative damage in Oryza sativa L. under thiocyanate exposure. Chemosphere, 243, 125472.
Liu, X., Tian, W., Kong, X., Jiang, M., Sun, X., & Lei, X. (2015). Selective removal of thiosulfate from thiocyanate-containing water by a three-dimensional structured adsorbent: A calcined NiAl-layered double hydroxide film. Rsc Advances, 5, 87948–87955.
Liu, Y., Yu, Z., Wang, Q., Zhu, X., Long, R., & Li, X. (2021). Application of sodium dodecyl sulfate intercalated Co-Al LDH composite materials (RGO/PDA/SDS-LDH) in membrane separation. Applied Clay Science, 209, 106138.
Liu, W., Yang, J., Cai, J., Li, H., Zhao, Y., Deng, X., Liu, Y., Mao, K., Meng, G., & Zhou, Y. (2022). Controllable synthesis of Co-Al layered double hydroxides with different anionic intercalation layers for the efficient removal of methyl orange. Environmental Technology, 44, 20–31.
Lu, Z.-H., Senosy, I. A., Zhou, D.-D., Yang, Z.-H., Guo, H.-M., & Liu, X. (2021). Synthesis and adsorption properties investigation of Fe3O4@ZnAl-LDH@MIL-53(Al) for azole fungicides removal from environmental water. Separation and Purification Technology, 276, 119282.
Maity, C. K., Sahoo, S., Verma, K., Behera, A. K., & Nayak, G. C. (2020). Facile functionalization of boron nitride (BN) for the development of high-performance asymmetric supercapacitors. New Journal of Chemistry, 44, 8106–8119.
Marcus, Y. (1997). Ion properties (vol. 54, pp. 2327–2334). CRC Press.
Mohanty, C., Das, N., Behera, A. K., & Tripathy, B. C. (2023). Efficiency of poly(m-aminophenol) as a novel adsorbent for individual/simultaneous removal of organic dyes and hexavalent chromium from water sources. Water, Air, & Soil Pollution, 234, 204.
Namasivayam, C., & Sangeetha, D. (2005). Kinetic studies of adsorption of thiocyanate onto ZnCl2 activated carbon from coir pith, an agricultural solid waste. Chemosphere, 60, 1616–1623.
Namasivayam, C., & Sureshkumar, M. V. (2007). Modelling thiocyanate adsorption onto surfactant-modified coir pith, an agricultural solid ‘waste.’ Process Safety and Environmental Protection, 85, 521–525.
Nazir, M. A., Khan, N. A., Cheng, C., Shah, S. S. A., Najam, T., Arshad, M., Sharif, A., Akhtar, S., & Rehman, A. U. (2020). Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Applied Clay Science, 190, 105564.
Noroozi, R., Al-Musawi, T. J., Kazemian, H., Kalhori, E. M., & Zarrabi, M. (2018). Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. Journal of Water Process Engineering, 21, 44–51.
Olson, G. J., Brierley, C. L., Briggs, A. P., & Calmet, E. (2006). Biooxidation of thiocyanate-containing refractory gold tailings from Minacalpa, Peru. Hydrometallurgy, 81, 159–166.
Oulego, P., Collado, S., Laca, A., & Diaz, M. (2017). Iron (II) as catalyst for thiocyanate wet oxidation: Mechanism and modelization. Chemical Engineering Journal, 316, 813–822.
Pan, J., Ma, J., Wu, H., Chen, B., He, M., Liao, C., & Wei, C. (2019). Application of metabolic division of labor in simultaneous removal of nitrogen and thiocyanate from wastewater. Water Research, 150, 216–224.
Pode, R., Popovici, E., Cocheci, L., Reisz, E., Seftel, E. M., & Pode, V. (2008). Sorption of phosphates and thiocyanates on isomorphic substituted Mg/Zn-Al-type hydrotalcites. Journal of the Serbian Chemical Society, 73, 835–843.
Raper, E., Stephenson, T., Fisher, R., Anderson, D. R., & Soares, A. (2019). Characterisation of thiocyanate degradation in a mixed culture activated sludge process treating coke wastewater. Bioresource Technology, 288, 121524.
Ravuru, S. S., Jana, A., & De, S. (2019). Synthesis of NiAl- layered double hydroxide with nitrate intercalation: Application in cyanide removal from steel industry effluent. Journal of Hazardous Materials, 373, 791–800.
Rodrigues, G. D., da Silva, M. D. C. H., da Silva, L. H. M., Paggioli, F. J., Minim, L. A., & dos Reis Coimbra, J. S. (2008). Liquid-liquid extraction of metal ions without use of organic solvent. Separation and Purification Technology, 62, 687–693.
Singh, H., & Mishra, B. K. (2018). Degradation of cyanide, aniline and phenol in pre-treated coke oven wastewater by peroxide assisted electro-oxidation process. Water Science and Technology, 78, 2214–2227.
Soltani, A., Ahmadian, N., Kanani, Y., Dehnokhalaji, A., & Mighani, H. (2012). Ab initio investigation of the SCN- chemisorption of single-walled boron nitride nanotubes. Applied Surface Science, 258, 9536–9543.
Temkin, M. I. (1941). Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. Zh Fiz Chim, 15, 296–332.
Turan, A., Keyikoglu, R., Kobya, M., & Khataee, A. (2020). Degradation of thiocyanate by electrochemical oxidation process in coke oven wastewater: Role of operative parameters and mechanistic study. Chemosphere, 255, 127014.
Vicente, J., & Diaz, M. (2003). Thiocyanate wet oxidation. Environmental Science & Technology, 37, 1452–1456.
Vu, H. P., & Moreau, J. W. (2015). Thiocyanate adsorption on ferrihydrite and its fate during ferrihydrite transformation to hematite and goethite. Chemosphere, 119, 987–993.
Wang, J., Han, Y., Li, J., & Wei, J. (2017). Selective adsorption of thiocyanate anions using straw supported ion imprinted polymer prepared by surface imprinting technique combined with RAFT polymerization. Separation and Purification Technology, 177, 62–70.
Wang, Z., Swanson, J. M. J., & Voth, G. A. (2018). Modulating the chemical transport properties of a transmembrane antiporter via alternative anion flux. Journal of the American Chemical Society, 140, 16535–16543.
Wang, J., Han, P., & Xia, Y. (2019). A cationic metal-organic framework for the highly efficient removal of thiocyanate from water. Microporous and Mesoporous Materials, 287, 107–113.
Wang, G., Li, Y., & Jin, Z. (2020). “Ship in a Bottle” design of ZIF-9@CoAl LDH hybrid compound as a high performance asymmetric supercapacitor. New Journal of Chemistry, 44, 7528–7540.
Wang, C., Xiong, C., He, Y., Yang, C., Li, X., Zheng, J., & Wang, S. (2021). Facile preparation of magnetic Zr-MOF for adsorption of Pb(II) and Cr(VI) from water: Adsorption characteristics and mechanisms. Chemical Engineering Journal, 415, 128923.
Wang, C., Liu, Y., Huang, M., Xiang, W., Wang, Z., Wu, X., Zan, F., & Zhou, T. (2022). A rational strategy of combining Fenton oxidation and biological processes for efficient nitrogen removal in toxic coking wastewater. Bioresource Technology, 363, 127897.
Wang, L., An, X., Xiao, X., Li, N., Xie, D., Lai, F., & Zhang, Q. (2023). Treatment of thiocyanate-containing wastewater: A critical review of thiocyanate destruction in industrial effluents. World Journal of Microbiology & Biotechnology, 39, 1–20.
Weber Walter, J., & Morris, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division, 89, 31–59.
Willemin, M.-E., & Lumen, A. (2016). Development of a PBPK model of thiocyanate in rats with an extrapolation to humans: A computational study to quantify the mechanism of action of thiocyanate kinetics in thyroid. Toxicology and Applied Pharmacology, 307, 19–34.
Willemin, M.-E., & Lumen, A. (2017). Thiocyanate: A review and evaluation of the kinetics and the modes of action for thyroid hormone perturbations. Critical Reviews in Toxicology, 47, 537–563.
Wu, T., Sun, D., Li, Y., Zhang, H., & Lu, F. (2011). Thiocyanate removal from aqueous solution by a synthetic hydrotalcite sol. Journal of Colloid and Interface Science, 355, 198–203.
Xi, B., & Shi, Q. (2013). Removement of thiocyanate from industrial wastewater by microwave-Fenton oxidation method. Journal of Environmental Sciences, 25, S201–S204.
Xie, F., Borowiec, J., & Zhang, J. (2013). Synthesis of AgCl nanoparticles-loaded hydrotalcite as highly efficient adsorbent for removal of thiocyanate. Chemical Engineering Journal, 223, 584–591.
Yu, X., Nishimura, F., & Hidaka, T. (2020). Anammox reactor exposure to thiocyanate: Long-term performance and microbial community dynamics. Bioresource Technology, 317, 123960.
Zhang, J., Chen, M., & Zhu, L. (2016). Activation of persulfate by Co3O4 nanoparticles for orange G degradation. Rsc Advances, 6, 758–768.
Zhao, Q., Ge, Y., Fu, K., Ji, N., Song, C., & Liu, Q. (2018a). Oxidation of acetone over Co-based catalysts derived from hierarchical layer hydrotalcite: Influence of Co/Al molar ratios and calcination temperatures. Chemosphere, 204, 257–266.
Zhao, X., Niu, C., Zhang, L., Guo, H., Wen, X., Liang, C., & Zeng, G. (2018b). Co-Mn layered double hydroxide as an effective heterogeneous catalyst for degradation of organic dyes by activation of peroxymonosulfate. Chemosphere, 204, 11–21.
Zhu, J., Zhu, Z., Zhang, H., Lu, H., & Qiu, Y. (2019). Calcined CoAl-layered double hydroxide as a heterogeneous catalyst for the degradation of acetaminophen and rhodamine B: Activity, stability, and mechanism. Environmental Science and Pollution Research, 26, 33329–33340.
Funding
This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA23030302) and Shangrao Major Technical Research Projects: Leading the Charge with Open Competition (No. 2021A001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Declarations
The manuscript is approved by all authors for publication. All the authors listed have approved the manuscript that is enclosed.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, J., Li, Z., Liu, X. et al. Thiocyanate Removal from Aqueous Solution by a Synthetic CoAl-Layered Double Hydroxide with Nitrate Intercalation. Water Air Soil Pollut 234, 579 (2023). https://doi.org/10.1007/s11270-023-06593-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06593-1