Abstract
Infectious bronchitis virus (IBV) and avian influenza virus (AIV) are two major respiratory infections in chickens. The coinfection of these viruses can cause significant financial losses and severe complications in the poultry industry across the world. To examine transcriptome profile changes during the early stages of infection, differential transcriptional profiles in tracheal tissue of three infected groups (i.e., IBV, AIV, and coinfected) were compared with the control group. Specific-pathogen-free chickens were challenged with Iranian variant-2-like IBV (IS/1494), UT-Barin isolates of H9N2 (A/chicken/Mashhad/UT-Barin/2017), and IBV-AIV coinfection; then, RNA was extracted from tracheal tissue. The Illumina RNA-sequencing (RNA-seq) technique was employed to investigate changes in the Transcriptome. Up- and downregulated differentially expressed genes (DEGs) were detected in the trachea transcriptome of all groups. The Kyoto Encyclopedia of Genes and Genomes pathway and Gene Ontology databases were examined to identify possible relationships between DEGs. In the experimental groups, upregulated genes were higher compared to downregulated genes. A more severe immune response was observed in the coinfected group; further, cytokine-cytokine receptor interaction, RIG-I-like receptor signaling, Toll-like receptor signaling, NOD-like receptor signaling, Janus kinase/signal transducer, and activator of transcription, and apoptotic pathways were important upregulated genes in this group. The findings of this paper may give a better understanding of transcriptome changes in the trachea during the early stages of infection with these viruses.






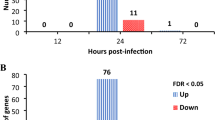
taken from the coinfected group (the error bars indicate 95% CI)
Similar content being viewed by others
References
Cook JK, Jackwood M, Jones R (2012) The long view: 40 years of infectious bronchitis research. Avian Pathol 41(3):239–250
Hassan KE, Shany SA, Ali A, Dahshan A-HM, Azza A, El-Kady MF (2016) Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poult Sci 95(6):1271–1280
Roussan D, Haddad R, Khawaldeh G (2008) Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poult Sci 87(3):444–448
Seifi S, Asasi K, Mohammadi A (2012) An experimental study on broiler chicken co-infected with the specimens containing avian influenza (H9subtype) and infectious bronchitis (4/91 strain) viruses. Iran J Vet Res 13(2):39
Fernandez-Siurob I, Retana M, Tellez G, Arroyo-Navarro L, Bañuelos-Hernandez B, Castellanos-Huerta I (2014) Assessment of viral interference using a chemical receptor blocker against avian influenza and establishment of protection levels in field outbreaks. Vaccine 32(11):1318–1322
Rahman SAE, El-Kenawy A, Neumann U, Herrler G, Winter C (2009) Comparative analysis of the sialic acid binding activity and the tropism for the respiratory epithelium of four different strains of avian infectious bronchitis virus. Avian Pathol 38(1):41–45
Westermann AJ, Gorski SA, Vogel J (2012) Dual RNA-seq of pathogen and host. Nat Rev Microbiol 10(9):618–630
Kameka AM, Haddadi S, Kim DS, Cork SC, Abdul-Careem MF (2014) Induction of innate immune response following infectious bronchitis corona virus infection in the respiratory tract of chickens. Virology 450:114–121
Zaffuto K, Estevez C, Afonso C (2008) Primary chicken tracheal cell culture system for the study of infection with avian respiratory viruses. Avian Pathol 37(1):25–31
Vervelde L, Matthijs M, Van Haarlem D, de Wit J, Jansen C (2013) Rapid NK-cell activation in chicken after infection with infectious bronchitis virus M41. Vet Immunol Immunopathol 151(3–4):337–341
Kruth SA (1998) Biological response modifiers: interferons, interleukins, recombinant products, liposomal products. Vet Clin N Am 28(2):269–295
Asif M, Lowenthal JW, Ford ME, Schat KA, Kimpton WG, Bean AG (2007) Interleukin-6 expression after infectious bronchitis virus infection in chickens. Viral Immunol 20(3):479–486
Guo X, Rosa AJ, Chen D-G, Wang X (2008) Molecular mechanisms of primary and secondary mucosal immunity using avian infectious bronchitis virus as a model system. Vet Immunol Immunopathol 121(3–4):332–343
Chhabra R, Chantrey J, Ganapathy K (2015) Immune responses to virulent and vaccine strains of infectious bronchitis viruses in chickens. Viral Immunol 28(9):478–488
Ghobadian Diali H, Hosseini H, Fallah Mehrabadi MH, Yahyaraeyat R, Ghalyanchilangeroudi A (2022) Evaluation of viral load and transcriptome changes in tracheal tissue of two hybrids of commercial broiler chickens infected with avian infectious bronchitis virus: a comparative study. Adv Virol. https://doi.org/10.1007/s00705-021-05322-5
Hashemi S, Ghalyanchilangeroudi A, Hosseini SM, Sheikhi N (2020) Comparative trachea transcriptome analysis between avian infectious bronchitis virus and avian pathogenic E. coli individual infection and co-infection in SPF chickens. Acta Virol 64(04):457–469. https://doi.org/10.4149/av_2020_413
Massin P, Deleage C, Oger A, Briand F-X, Quenault H, Blanchard Y (2013) Differential cellular gene expression in duck trachea infected with a highly or low pathogenic H5N1 avian influenza virus. Virol J. https://doi.org/10.1186/1743-422x-10-279
Najafi H, Langeroudi AG, Hashemzadeh M, Karimi V, Madadgar O, Ghafouri SA, Maghsoudlo H, Farahani RK (2016) Molecular characterization of infectious bronchitis viruses isolated from broiler chicken farms in Iran, 2014–2015. Adv Virol 161(1):53–62
Mehrabadi MHF, Ghalyanchilangeroudi A, Ghafouri SA, Malekan M, Ziafati Z, Hosseini H, Mousavi FS, Jabbarifakhr M, Aghaeean L (2019) Full-genome characterization and genetic analysis of a H9N2 virus in commercial broilers in Iran, 2017. Trop Anim Health Prod 51(6):1737–1749
Reed LJ, Muench H (1938) A simple method of estimating fifty per cent endpoints. Am J Epidemiol 27(3):493–497
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21
Huang DW, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37(1):1–13
Kanehisa M, Sato Y, Furumichi M, Morishima K, Tanabe M (2019) New approach for understanding genome variations in KEGG. Nucleic Acids Res 47(D1):D590–D595
Paul MS, Brisbin JT, Abdul-Careem MF, Sharif S (2013) Immunostimulatory properties of Toll-like receptor ligands in chickens. Vet Immunol Immunopathol 152(3–4):191–199
Chen S, Cheng A, Wang M (2013) Innate sensing of viruses by pattern recognition receptors in birds. Vet Res 44(1):1–12
Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M (2005) Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem 280(7):5571–5580
Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M (2007) Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol 178(6):3368–3372
Karpala AJ, Lowenthal JW, Bean AG (2012) Identifying innate immune pathways of the chicken may lead to new antiviral therapies. Vet Immunol Immunopathol 148(1–2):100–109
Jensen S, Thomsen AR (2012) Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol 86(6):2900–2910
Barjesteh N, O’Dowd K, Vahedi SM (2020) Antiviral responses against chicken respiratory infections: Focus on avian influenza virus and infectious bronchitis virus. Cytokine 127:154961
Gack MU, Shin YC, Joo C-H, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S (2007) TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446(7138):916–920
Khatri M, Sharma JM (2006) Infectious bursal disease virus infection induces macrophage activation via p38 MAPK and NF-κB pathways. Virus Res 118(1–2):70–77
Fulton R, Thacker H, Reed W, DeNicola D (1997) Effect of cytoxan®-induced heteropenia on the response of specific-pathogen-free chickens to infectious bronchitis. Avian Dis 41:511–518
Matthijs MG, Ariaans MP, Dwars RM, van Eck JH, Bouma A, Stegeman A, Vervelde L (2009) Course of infection and immune responses in the respiratory tract of IBV infected broilers after superinfection with E. coli. Vet Immunol Immunopathol 127(1–2):77–84
Ariaans MP, Matthijs MG, van Haarlem D, van de Haar P, van Eck JH, Hensen EJ, Vervelde L (2008) The role of phagocytic cells in enhanced susceptibility of broilers to colibacillosis after infectious bronchitis virus infection. Vet Immunol Immunopathol 123(3–4):240–250
He H, Genovese KJ, Kogut MH (2011) Modulation of chicken macrophage effector function by TH1/TH2 cytokines. Cytokine 53(3):363–369
Crowley TM, Haring VR, Moore R (2011) Chicken anemia virus: an understanding of the in-vitro host response over time. Viral Immunol 24(1):3–9
Hay S, Kannourakis G (2002) A time to kill: viral manipulation of the cell death program. J Gen Virol 83(7):1547–1564
Li FQ, Tam JP, Liu DX (2007) Cell cycle arrest and apoptosis induced by the coronavirus infectious bronchitis virus in the absence of p53. Virology 365(2):435–445
Cong F, Liu X, Han Z, Shao Y, Kong X, Liu S (2013) Transcriptome analysis of chicken kidney tissues following coronavirus avian infectious bronchitis virus infection. BMC Genomics 14(1):1–13
Matsumoto M, Seya T (2008) TLR3: interferon induction by double-stranded RNA including poly (I: C). Adv Drug Deliv Rev 60(7):805–812
Huang Q, Wang K, Pan L, Qi K, Liu H, Chen H (2017) Co-infection of H9N2 subtype avian influenza virus and infectious bronchitis virus decreases SP-A expression level in chickens. Vet Microbiol 203:110–116
Acknowledgements
The authors would like to express their gratitude to the Sheikh Bahaei National High-Performance Computing Center (SBNHPCC) for donating computing resources and time. SBNHPCC is supported by the Scientific and Technological Department of the Presidential Office and the Isfahan University of Technology.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. SHZ, AG, and AK performed material preparation, data collection, and analysis. SHZ wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors approved the final manuscript for publication. In addition, the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
No humans were used in the present study. Ethical considerations were observed following the principles of the University of Tehran under sterile conditions. In addition, the study protocol was approved by the Ethics Committee of Tehran University of Medical Sciences (code: UT1397612).
Additional information
Edited by William Dundon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zamzam, S.H., Ghalyanchilangeroudi, A. & Khosravi, A.R. Comparative trachea transcriptome analysis in SPF broiler chickens infected with avian infectious bronchitis and avian influenza viruses. Virus Genes 58, 203–213 (2022). https://doi.org/10.1007/s11262-022-01893-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-022-01893-w