Abstract
Purpose
The NAD+-dependent deacetylase, sirtuin 1 (SIRT1), plays an important role in vascular calcification induced by high glucose and/or high phosphate levels. However, the mechanism by which SIRT1 regulates this process is still not fully understood. Thus, this study aimed to determine the role of high glucose and phosphate in vascular calcification and the molecular mechanisms underlying SIRT1 regulation.
Methods
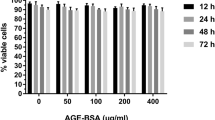
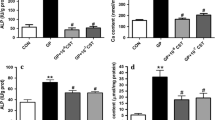
Vascular smooth muscle cells (VSMCs) were cultured under normal, high phosphate, and/or high-glucose conditions for 9 days. Alizarin red staining and calcification content analyses were used to determine calcium deposition. VSMC senescence was detected by β-galactosidase (SA-β-Gal) staining and p21 expression.
Results
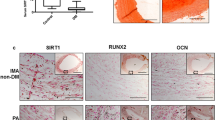
Mouse VSMCs exposed to high phosphate and high glucose in vitro showed increased calcification, which was correlated with the induction of cell senescence, as confirmed by the increased SA-β-galactosidase activity and p21 expression. SRT1720, an activator of SIRT1, inhibits p65 acetylation, the nuclear factor-κ-gene binding (NF-κB) pathway, and VSMC transdifferentiation, prevents senescence and reactive oxygen species (ROS) production, and reduces vascular calcification. In contrast, sirtinol, an inhibitor of SIRT1, increases p65 acetylation, activates the NF-κB pathway, induces vascular smooth muscle cell transdifferentiation and senescence, and promotes vascular calcification.
Conclusions
High glucose and high phosphate levels induce senescence and vascular calcification in VSMCs, and the combined effect of high glucose and phosphate can inhibit SIRT1 expression. SIRT1 inhibits vascular smooth muscle cell senescence and osteogenic differentiation by inhibiting NF-κB activity, thereby inhibiting vascular calcification.







Similar content being viewed by others
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Abbreviations
- CKD:
-
Chronic kidney disease
- DM:
-
Diabetes mellitus
- VSMCs:
-
Vascular smooth muscle cells
- NF-κB:
-
Nuclear factor-κ-gene binding
- α-SMA:
-
Alpha smooth muscle actin
- SM22α:
-
Smooth muscle 22 alpha
- RUNX2:
-
Runt-related transcription factor-2
- Pit-1:
-
Type III sodium‑dependent phosphate cotransporter-1
- BMP2:
-
Bone morphogenetic protein 2
- VC:
-
Vascular calcification
- SA-β-Gal:
-
Senescence-associated-β-galactosidase
- SIRT1:
-
Sirtuin 1
- AcP65:
-
Acetylated-p65
- ROS:
-
Reactive oxygen species
- ESRD:
-
End stage renal disease
- VC:
-
Vascular calcification
References
Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY (2018) Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res 114:622–634. https://doi.org/10.1093/cvr/cvy007
Voelkl J, Luong TTD, Tuffaha R, Musculus K, Auer T, Lian X, Daniel C, Zickler D, Boehme B, Sacherer M, Metzler B, Kuhl D, Gollasch M, Amann K, Müller DN, Pieske B, Lang F, Alesutan I (2018) SGK1 induces vascular smooth muscle cell calcification through NF-κB signaling. J Clin Investig 128:3024–3040. https://doi.org/10.1172/JCI96477
Yoshida T, Yamashita M, Horimai C, Hayashi M (2017) Smooth muscle-selective nuclear factor-κB inhibition reduces phosphate-induced arterial medial calcification in mice with chronic kidney disease. J Am Heart Assoc. https://doi.org/10.1161/JAHA.117.007248
Russo D, Morrone LFP, Imbriaco M, Pota A, Russo L, Scognamiglio B, Sorrent R (2013) Coronary artery calcification and outcomes in diabetic patients with and without chronic kidney disease. Blood Purif 36:17–20. https://doi.org/10.1016/j.jacc.2003.09.068
Raggi P, Shaw LJ, Berman DS, Callister TQ (2004) Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 43:1663–1669. https://doi.org/10.1016/j.jacc.2003.09.068
Hwang JS, Ham SA, Yoo T, Lee WJ, Paek KS, Lee CH, Seo HG (2016) Sirtuin 1 mediates the actions of peroxisome proliferator-activated receptor delta on the oxidized low-density lipoprotein-triggered migration and proliferation of vascular smooth muscle cells. Mol Pharmacol 90:522–529. https://doi.org/10.1124/mol.116.104679
Ma S, Fan L, Cao F (2019) Combating cellular senescence by sirtuins: implications for atherosclerosis. Biochim Biophys Acta Mol Basis Dis 1865:1822–1830. https://doi.org/10.1016/j.bbadis.2018.06.011
Khan M, Shah SA, Kim MO (2017) 17β-Estradiol via SIRT1/Acetyl-p53/NF-kB signaling pathway rescued postnatal rat brain against acute ethanol intoxication. Mol Neurobiol 55:3067–3078. https://doi.org/10.1007/s12035-017-0520-8
Takemura A, Iijima K, Ota H, Son BK, Ito Y, Ogawa S, Eto M, Akishita M, Ouchi Y (2011) Sirtuin 1 retards hyperphosphatemia-induced calcification of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 31:2054–2062. https://doi.org/10.1161/ATVBAHA.110.216739
Bartoli-Leonard F, Wilkinson FL, Schiro A, Inglott FS, Alexander MY, Weston R (2019) Suppression of SIRT1 in diabetic conditions induces osteogenic differentiation of human vascular smooth muscle cells via RUNX2 signalling. Sci Rep 31:2054–2062. https://doi.org/10.1038/s41598-018-37027-2
Xu D, Zeng F, Han L, Wang J, Yin Z, Lv L, Guo L, Wang D, Xu Y, Zhou H (2019) The synergistic action of phosphate and interleukin-6 enhances senescence-associated calcification in vascular smooth muscle cells depending on p53. Mech Ageing Dev 182:111–124. https://doi.org/10.1016/j.mad.2019.111124
Bessueille L, Fakhry M, Hamade E, Badran B, Magne D (2015) Glucose stimulates chondrocyte differentiation of vascular smooth muscle cells and calcification: a possible role for IL-1beta. FEBS Lett 589:2797–2804. https://doi.org/10.1016/j.febslet.2015.07.045
Henaut L, Sanchez-Nino MD, Castillo GA-E, Sanz AB, Ortiz A (2016) Targeting local vascular and systemic consequences of inflammation on vascular and cardiac valve calcification. Expert Opin Ther Targets 20:89–105. https://doi.org/10.1517/14728222.2015.1081685
Desjardins MP, Sidibe A, Fortier C, Mac-Way F, Marquis K, De Serres S, Lariviere R, Agharazii M (2018) Association of interleukin-6 with aortic stiffness in end-stage renal disease. J Am Soc Hypertens 12:5–13. https://doi.org/10.1016/j.jash.2017.09.013
Chen Y, Liu H, Zhang H, Liu E, Xu CB, Su X (2016) The sirt1/NF-kB signaling pathway is involved in regulation of endothelin type B receptors mediated by homocysteine in vascular smooth muscle cells. Biomed Pharmacother 84:1979–1985. https://doi.org/10.1016/j.biopha.2016.11.011
Louvet L, Bazin D, Buchel J, Steppan S, Passlick-Deetjen J, Massy ZA (2015) Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PLoS ONE 10:e0115342. https://doi.org/10.1371/journal.pone.0115342
MacRae (2011) MOVAS-1 cell line: a new in vitro model of vascular calcification. Int J Mol Med 27:663–668. https://doi.org/10.3892/ijmm.2011.631
Kim DH, Lee KC, Han SY (2018) Cyclosporin A aggravates calcification of vascular smooth muscle cells under high-glucose conditions with a calcifying medium. Ann Transplant 23:112–118. https://doi.org/10.12659/aot.908168
Belmokhtar K, Ortillon J, Jaisson S, Massy ZA, Rombi CB, Doue M, Maurice P, Fritz G, Gillery P, Schmidt AM, Rieu P, Toure F (2019) Receptor for advanced glycation end products: a key molecule in the genesis of chronic kidney disease vascular calcification and a potential modulator of sodium phosphate co-transporter PIT-1 expression. Nephrol Dial Transplant 34:2018–2030. https://doi.org/10.1093/ndt/gfz012
Yao L, Sun YT, Sun W, Xu TH, Ren C, Fan X, Sun L, Liu LL, Feng JM, Ma JF, Wang LN (2015) High phosphorus level leads to aortic calcification via beta-catenin in chronic kidney disease. Am J Nephrol 41:28–36. https://doi.org/10.1159/000370250
Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P (2017) Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol 13:429–442. https://doi.org/10.1038/nrneph.2017.60
Albright RA, Stabach P, Cao W, Kavanagh D, Mullen I, Braddock AA, Covo MS, Tehan M, Yang G, Cheng Z, Bouchard K, Yu ZX, Thorn S, Wang X, Folta-Stogniew EJ, Negrete A, Sinusas AJ, Shiloach J, Zubal G, Madri JA, De La Cruz EM, Braddock DT (2015) ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nat Commun 6:10006. https://doi.org/10.1038/ncomms10006
Cano-Megias M, Bouarich H, Guisado-Vasco P, Fernandez MP, de Fuente GA, Alvarez-Sanz C, Rodriguez-Puyol D (2019) Coronary artery calcification in patients with diabetes mellitus and advanced chronic kidney disease. Endocrinol Diabetes Nutr 66:297–304. https://doi.org/10.1016/j.endinu.2018.09.003
Zhang Z, Lin J, Nisar M, Chen T, Xu T, Zheng G, Wang C, Jin H, Chen J, Gao W, Tian N, Wang X, Zhang X (2019) The Sirt1/P53 axis in diabetic intervertebral disc degeneration pathogenesis and therapeutics. Oxid Med Cell Longev 2019:1–21. https://doi.org/10.1155/2019/7959573
Zhang P, Li Y, Du Y, Li G, Wang L, Zhou F (2016) Resveratrol ameliorated vascular calcification by regulating Sirt-1 and Nrf2. Transplant Proc 48:3378–3386. https://doi.org/10.1016/j.transproceed.2016.10.023
Willems BA, Furmanik M, Caron MMJ, Chatrou MLL, Kusters DHM, Welting TJM, Stock M, Rafael MS, Viegas CSB, Simes DC, Vermeer C, Reutelingsperger CPM, Schurgers LJ (2018) Ucma/GRP inhibits phosphate-induced vascular smooth muscle cell calcification via SMAD-dependent BMP signalling. Sci Rep 8:4961. https://doi.org/10.1038/s41598-018-23353-y
Mokas S, Lariviere R, Lamalice L, Gobeil S, Cornfield DN, Agharazii M, Richard DE (2016) Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int 90:598–609. https://doi.org/10.1038/s41598-018-23353-y
Mistry Y, Poolman T, Williams B, Herbert KE (2013) A role for mitochondrial oxidants in stress-induced premature senescence of human vascular smooth muscle cells. Redox Biol 1:411–417. https://doi.org/10.1016/j.redox.2013.08.004
Kim GD (2019) SIRT1-mediated protective effect of Aralia elata (Miq.) seem against high-glucose-induced senescence in human umbilical vein endothelial cells. Nutrients 11:2625. https://doi.org/10.3390/nu11112625
Wang X, Gao Y, Tian N, Wang T, Shi Y, Xu J, Wu B (2019) Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT–NF-κB p65 axis. Sci Rep 9:323. https://doi.org/10.1038/s41598-018-36911-1
Bagul PK, Deepthi N, Sultana R, Banerjee SK (2015) Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J Nutr Biochem 26:1298–1307. https://doi.org/10.1016/j.jnutbio.2015.06.006
Li S, Zhan J-K, Wang Y-J, Lin X, Zhong J-Y, Wang Y, Tan P, He J-Y, Cui X-J, Chen Y-Y, Huang W, Liu Y-S (2019) Exosomes from hyperglycemia-stimulated vascular endothelial cells contain versican that regulate calcification/senescence in vascular smooth muscle cells. Cell Biosci 9:1. https://doi.org/10.1186/s13578-018-0263-x
Zhang D, Bi X, Liu Y, Huang Y, Xiong J, Xu X, Xiao T, Yu Y, Jiang W, Huang Y, Zhang J, Zhang B, Zhao J (2017) High phosphate-induced calcification of vascular smooth muscle cells is associated with the TLR4/NF-kappab signaling pathway. Kidney Blood Press Res 42:1205–1215. https://doi.org/10.1159/000485874
Wang P, Quan Z, Luo D, Chen W, Peng D (2019) Spironolactone dosedependently alleviates the calcification of aortic rings cultured in hyperphosphatemic medium with or without hyperglycemia by suppressing phenotypic transition of VSMCs through downregulation of Pit1. Mol Med Rep 19:3622–3632. https://doi.org/10.3892/mmr.2019.10039
Lau WL, Festing MH, Giachelli CM (2010) Phosphate and vascular calcification: emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb Haemost 104:464–470. https://doi.org/10.1160/TH09-12-0814
Beck L, Leroy C, Salaün C, Margall-Ducos G, Desdouets C, Friedlander G (2009) Identification of a novel function of PiT1 critical for cell proliferation and independent of its phosphate transport activity. J Biol Chem 284:31363–31374. https://doi.org/10.1074/jbc.M109.053132
Kimata M, Michigami T, Tachikawa K, Okada T, Koshimizu T, Yamazaki M, Kogo M, Ozono K (2010) Signaling of extracellular inorganic phosphate up-regulates cyclin D1 expression in proliferating chondrocytes via the Na+/Pi cotransporter Pit-1 and Raf/MEK/ERK pathway. Bone 47:938–947. https://doi.org/10.1016/j.bone.2010.08.006
Koumakis E, Millet-Botti J, Benna JE, Leroy C, Boitez V, Codogno P, Friedlander G, Forand A (2019) Novel function of PiT1/SLC20A1 in LPS-related inflammation and wound healing. Sci Rep 9:1808. https://doi.org/10.1038/s41598-018-37551-1
Wang P, Zhou P, Chen W, Peng D (2019) Combined effects of hyperphosphatemia and hyperglycemia on the calcification of cultured human aortic smooth muscle cells. Exp Ther Med 17:863–868. https://doi.org/10.1038/s41598-018-37551-1
Yoshida T, Yamashita M, Horimai C (2013) High glucose concentration does not modulate the formation of arterial medialcalcification in experimental uremic rats. J Vasc Res 50:512–520. https://doi.org/10.1159/000355263
Funding
This work was supported by grants from the Natural Science Foundation of Anhui Province (No. 2008085MH244) and the National Natural Science Foundation of China in the Second Hospital of Anhui Medical University (No. 2020GMFY04).
Author information
Authors and Affiliations
Contributions
HZ and DW conceived and designed the experiments. MZ, TL, ZT, YZ, XW and DZ performed the experiments. MZ, HZ, and DW interpreted the data that contributed to the manuscript structure and flow. YF, FH, and MN collected the experimental specimens. MZ, DW, and HZ wrote the manuscript. All authors reviewed and confirmed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, M., Li, T., Tu, Z. et al. Both high glucose and phosphate overload promote senescence-associated calcification of vascular muscle cells. Int Urol Nephrol 54, 2719–2731 (2022). https://doi.org/10.1007/s11255-022-03195-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03195-4