Abstract
Objective
The present study aimed to investigate the effects of an imbalance in the estrogen/androgen ratio on prostate fibrosis.
Methods
Different concentrations of dihydrotestosterone (DHT) or estradiol (E2) dissolved in corn oil were injected subcutaneously into the nape of the castrated Sprague–Dawley (SD) rats over 28 consecutive days. Masson’s trichrome staining and immunohistochemical staining were performed to detect the content of collagen fibers and the expression of collagen I, fibronectin, and elastin in the rat prostate of each group, respectively. DHT + E2 at different concentrations was administered to human normal prostate stromal immortalized cells (WPMY-1 cells) for 1 week. The expression of collagen I, fibronectin, elastin, transforming growth factor-β1 (TGF-β1), Smad3, and Smad7 was detected by Western blotting (WB). Then, WPMY-1 cells treated with 10 nM DHT + 5 pM E2 were incubated with the TGF-β/Smad pathway inhibitor SD208 for 1 week, after which collagen I, fibronectin, and elastin expression was detected by WB.
Results
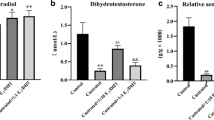
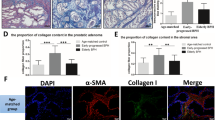
Compared with the uncastrated control and corn oil injection groups, the collagen fiber content and collagen I and fibronectin expression were increased and elastin expression was decreased in the castrated rat prostate with corn oil injection group (p < 0.01). Compared to castrated corn oil injection group, collagen fiber content, collagen I, and fibronectin expression were significantly decreased, and elastin expression was significantly increased in the castrated rat prostate 0.15 mg/kg DHT treatment group (p < 0.01). Following treatment with 0.15 mg/kg DHT, the content of collagen fibers, and the expression of collagen I and fibronectin were increased, and the expression of elastin was decreased in the rat prostate with increasing concentrations of E2 treatment group compared to the 0.15 mg/kg DHT group (p < 0.05, p < 0.01). Following treatment with 0.05 mg/kg E2, the collagen fiber content and the expression of collagen I and fibronectin were decreased, and the expression of elastin was increased in the rat prostate with increasing DHT concentration treatment group compared to the 0.05 mg/kg E2 group (p < 0.05, p < 0.01). Compared with the Control group, the expression of collagen I, fibronectin, TGF-β1 and Smad3 was decreased, and the expression of elastin and Smad7 was increased in WPMY-1 cells after treatment with 10 nM DHT (p < 0.01). Following treatment with 10 nM DHT, the expression of collagen I, fibronectin, TGF-β1, and Smad3 was increased, and the expression of elastin and Smad7 was decreased in WPMY-1 cells with increasing E2 concentration treatment compared to the 10 nM DHT group (p < 0.05, p < 0.01). Following treatment with 5 pM E2, the expression of collagen I, fibronectin, TGF-β1, and Smad3 was decreased, and elastin and Smad7 expression was increased with increasing DHT concentration compared to the 5 pM E2 group (p < 0.05, p < 0.01). Compared to the 10 nM DHT + 5 pM E2 group, the expressions of collagen I and fibronectin were decreased; the expression of elastin was increased in WPMY-1 cells after the supplement of TGF-β/Smad pathway inhibitor SD208 group (p < 0.05, p < 0.01).
Conclusions
An imbalance in the estrogen/androgen ratio may affect prostate fibrosis. E2 may activate the degree of prostate fibrosis. In contrast to the effect of E2, DHT may inhibit the degree of prostate fibrosis, which might involve the TGF-β/Smad signaling pathway.




Similar content being viewed by others
References
Eckhardt MD, van Venrooij GE, Boon TA (2001) Symptoms and quality of life versus age, prostate volume, and urodynamic parameters in 565 strictly selected men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology 57(4):695–700
Bushman W (2009) Etiology, epidemiology, and natural history of benign prostatic hyperplasia. Urol Clin North Am 36(4):403–415
Agrawal CS, Chalise PR, Bhandari BB (2008) Correlation of prostate volume with international prostate symptom score and quality of life in men with benign prostatic hyperplasia. Nepal Med Coll J 10(2):104–107
Simon RM, Howard LE, Moreira DM et al (2016) does prostate size predict the development of incident lower urinary tract symptoms in men with mild to no current symptoms? Results from the REDUCE trial. Eur Urol 69(5):885–891
Cantiello F, Cicione A, Salonia A et al (2013) Periurethral fibrosis secondary to prostatic inflammation causing lower urinary tract symptoms: a prospective cohort study. Urology 81(5):1018–1024
Ma J, Gharaee-Kermani M, Kunju L et al (2012) Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol 188(4):1375–1381
Asiedu B, Anang Y, Nyarko A et al (2017) The role of sex steroid hormones in benign prostatic hyperplasia. Aging Male 20(1):1–6
Enatsu N, Chiba K, Sumii K et al (2017) Dutasteride-mediated morphological changes in the genitourinary tract associated with altered expression patterns of the androgen and estrogen receptors in male rats. Andrology 5(2):347–353
Roznovanu SL, Radulescu D, Novac C et al (2005) The morphologic changes induced by hormone and radiation therapy on prostate carcinoma. Rev Med Chir Soc Med Nat Iasi 109(2):337–342
Vignozzi L, Morelli A, Sarchielli E et al (2012) Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J Endocrinol 212(1):71–84
Katoh M (2018) Multi-layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β-catenin signaling activation (Review). Int J Mol Med 42(2):713–725
Zhou Y, Xiao X-Q, Chen L-F et al (2009) Proliferation and phenotypic changes of stromal cells in response to varying estrogen/androgen levels in castrated rats. Asian J Androl 11(4):451–459
Wang C, Xiaoling Du, Yang R et al (2015) The therapeutic effects of docosahexaenoic acid on oestrogen/androgen-induced benign prostatic hyperplasia in rats. J Steroid Biochem Mol Biol 145:28–37
Cao Y, Xiao Y, Ravid R, Guan ZZ (2010) Changed clathrin regulatory proteins in the brains of Alzheimer’s disease patients and animal models. J Alzheimers Dis 22(1):329–342
Shi X, Peng Y, Du X et al (2017) Estradiol promotes epithelial-to-mesenchymal transition in human benign prostatic epithelial cells. Prostate 77(14):1424–1437
Chen J, Rong N, Liu M et al (2021) The exosome-like vesicles derived from androgen exposed-prostate stromal cells promote epithelial cells proliferation and epithelial-mesenchymal transition. Toxicol Appl Pharmacol 411:115384
Cunha GR (1994) Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer 74(3 Suppl):1030–1044
Derynck R, Budi EH (2019) Specificity, versatility, and control of TGF-β family signaling. Sci Signal 12(570):5183
Cao Y, Luo GH, Luo L et al (2015) Re-epithelialization resulted from prostate basal cells in canine prostatic urethra may represent the ideal healing method after two-micron laser resection of the prostate. Asian J Androl 17(5):831–838
Chatterjee A, Kosmacek EA, Oberley-Deegan RE (2017) MnTE-2-PyP treatment, or NOX4 inhibition, protects against radiation-induced damage in mouse primary prostate fibroblasts by inhibiting the TGF-Beta 1 signaling pathway. Radiat Res 187(3):367–381
Acknowledgements
This study was supported by the grants from the National Natural Science Foundation of China (No. 81860141 and 82160149) and Guizhou Provincial Science and Technology Foundation (No. [2021]378).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, Y., Tian, Y., Zhang, H. et al. Imbalance in the estrogen/androgen ratio may affect prostate fibrosis through the TGF-β/Smad signaling pathway. Int Urol Nephrol 54, 499–508 (2022). https://doi.org/10.1007/s11255-021-03079-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-03079-z