Abstract
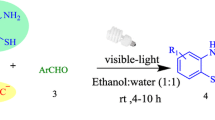
The development of environmentally benign protocols to synthesize novel N-heterocycles is vital in the field of synthetic organic chemistry. We herein report a successful one-pot domino synthesis of novel 6,7 disubstituted 1H-pyrroles using substituted phenacyl bromide, barbituric acid/Meldrums acid, aromatic amines catalysed by 5 mol% Fluorescein in presence of visible light. This procedure is a useful and adaptable method for the synthesis of pyrroles since it is compatible with a wide range of sensitive functional groups, does not require column chromatography purification. During the reaction, Fluorescein may catalyse the formation of enamine leading to amino alcohol which subsequently undergoes dehydration to give 6,7 disubstituted 1H-pyrroles. All the synthesized derivatives were obtained in 90–95% yields and were characterized by 1H, 13C NMR and HRMS (ESI) analysis.
Graphical Abstract








Similar content being viewed by others
References
Wang F, Sikma E, Duan Z, Sarma T, Lei C, Zhang Z, Humphrey SM, Sessler JL (2019) Shape-persistent pyrrole-based covalent organic cages: synthesis, structure and selective gas adsorption properties. Chem Commun 55:6185–6188
Jadoun S, Biswal L, Riaz U (2018) Tuning the optical properties of poly (o-phenylenediamine-co-pyrrole) via template mediated copolymerization. Des Monomers Polym 21:75–81
Ninis O, Kacimi R, Bouaamlat H, Abarkan M, Bouachrine M (2017) Theoretical studies of photovoltaic properties for design of new azo-pyrrole photo-sensitizer materials as dyes in solar cells. J Mater Environ Sci 8:2572–2578
Ahmad S, Alam O, Naim MJ, Shaquiquzzaman M, Alam MM, Iqbal M (2018) Pyrrole: an insight into recent pharmacological advances with structure activity relationship. Eur J Med Chem 157:527–561
Fatahala SS, Nofal S, Mahmoud E, Abd El-hameed RH (2019) Pyrrolopyrazoles: synthesis, evaluation and pharmacological screening as antidepressant agents. Med Chem 15:911–922
Battilocchio C, Poce G, Alfonso S, Porretta GC, Consalvi S, Sautebin L, Pace S, Rossi A, Ghelardini C, Mannelli LDC, Schenone S, Giordani A, Francesco LD, Patrignani P, Biava M (2013) A class of pyrrole derivatives endowed with analgesic/anti-inflammatory activity. Bioorganic Med Chem 21:3695–3701
Harrak Y, Rosell G, Daidone G, Plescia S, Schillaci D, Pujol MD (2007) Synthesis and biological activity of new anti-inflammatory compounds containing the 1, 4-benzodioxine and/or pyrrole system. Bioorganic Med Chem 15:4876–4890
Ghorab MM, Ragab FA, Heiba HI, Youssef HA, El-Gazzar MG (2010) Synthesis of novel pyrrole and pyrrolo [2 3-d] pyrimidine derivatives bearing sulfonamide moiety for evaluation as anticancer and radiosensitizing agents. Bioorganic Med Chem Lett 20:6316–6320
Pegklidou K, Papastavrou N, Gkizis P, Komiotis D, Balzarini J, Nicolaou I (2015) N-substituted pyrrole-based scaffolds as potential anticancer and antiviral lead structures. Med Chem 11:602–608
Yao TT, Xiao DX, Li ZS, Cheng JL, Fang SW, Du YJ, Zhao JH, Dong XW, Zhu GN (2017) Design, synthesis, and fungicidal evaluation of novel pyrazole-furan and pyrazole-pyrrole carboxamide as succinate dehydrogenase inhibitors. J Agric Food Chem 65:5397–5403
Ye Z, Shi L, Shao X, Xu X, Xu Z, Li Z (2013) Pyrrole-and dihydropyrrole-fused neonicotinoids: design, synthesis, and insecticidal evaluation. J Agric Food Chem 61:312–319
Boukouvala MC, Kavallieratos NG, Athanassiou CG, Hadjiarapoglou LP (2016) Insecticidal effect of two novel pyrrole derivatives against two major stored product insect species. Crop Prot 84:1–7
Rico R, Bermejo F (1995) Total synthesis of (−)-ampullicin and (+)-isoampullicin two growth regulators from Ampulliferina Sp no 27. Tetrahedron Lett 36:7889–7892
Bianco MDCAD, Marinho DILF, Hoelz LVB, Bastos MM, Boechat N (2021) Pyrroles as privileged scaffolds in the search for new potential HIV inhibitors. Pharmaceuticals 14:893
Williams IS, Joshi P, Gatchie L, Sharma M, Satti NK, Vishwakarma RA, Chaudhuri B, Bharate SB (2017) Synthesis and biological evaluation of pyrrole-based chalcones as CYP1 enzyme inhibitors for possible prevention of cancer and overcoming cisplatin resistance. Bioorganic Med Chem Lett 27:3683–3687
Wu C, Wang W, Fang L, Su W (2018) Programmable pyrrole-imidazole polyamides: a potent tool for DNA targeting. Chin Chem Lett 29:1105–1112
Peifer C, Selig R, Kinkel K, Ott D, Totzke F, Schächtele C, Heidenreich R, Röcken M, Schollmeyer D, Laufer S (2008) Design, synthesis, and biological evaluation of novel 3-aryl-4-(1 H-indole-3yl)-1, 5-dihydro-2 H-pyrrole-2-ones as vascular endothelial growth factor receptor (VEGF-R) inhibitors. J Med Chem 51:3814–3824
Zarrouk A, Hammouti B, Lakhlifi T, Traisnel M, Vezin H, Bentiss F (2015) New 1H-pyrrole-2, 5-dione derivatives as efficient organic inhibitors of carbon steel corrosion in hydrochloric acid medium: electrochemical XPS and DFT studies. Corros Sci 90:572–584
Ash M (2004) Handbook of preservatives. Synapse info resources.
Xu Y, Wang Z, Gan Z, Xi Q, Duan Z, Mathey F (2015) Versatile synthesis of phospholides from open-chain precursors application to annelated pyrrole–and silole–phosphole rings. Org Lett 17:1732–1734
Fan H, Peng J, Hamann MT, Hu JF (2008) Lamellarins and related pyrrole-derived alkaloids from marine organisms. Chem Rev 108:264–287
Vinayak A, Sudha M, Lalita SK (2017) Design synthesis and characterization of novel amine derivatives of 5-[5-(chloromethyl)-1, 3, 4-oxadiazol-2-yl]- 2-(4-fluorophenyl)-pyridine as a new class of anticancer agents Dhaka Uni. J Pharm Sci 16:11–19
Vinayak A, Sudha M, Jagadeesha AH, Lalita SK (2015) Design, synthesis, characterization and cancer cell growth-inhibitory properties of novel derivatives of 2-(4-fluoro-phenyl)-5-(5-aryl substituted-1, 3, 4-oxadiazol-2-yl) pyridine. Br J Pharm Res 7:34–43
Vinayak A, Sudha M, Lalita SK, Rao Prakash K (2014) Design, synthesis, characterization and anticancer properties of novel 2-chloro-N-(aryl substituted) acetamide derivatives of 5-[2-(4-methoxyphenyl) pyridin-3-yl]-1, 3, 4-oxadiazole-2-thiol. Int J Drug Dev Res 6:188–195
Vinayak A, Sudha M, Jaadeesha AH, Kulkarni P, Lalita KS, Rao PK (2014) Synthesis, characterization of some novel 1, 3, 4-oxadiazole compounds containing 8-hydroxy quinolone moiety as potential antibacterial and anticancer agents. Int J Pharm Res 4(4):180–185
Vinayak A (2014) Design, synthesis and cytotoxic evaluation of novel 2-(4-N, N-dimethyl) pyridine containing 1, 3, 4-oxadiazole moiety. Asian J Biomed Pharm Sci 4:1–5
Vinayak A, Sudha M, Lalita KS, Rao Prakash K (2014) Design, synthesis, characterization and anticancer properties of novel derivatives of 1-[2-(Aryl substituted)-5- (4’-methoxy-biphenyl-4-yl)-[1, 3, 4] oxadiazole-3-yl]- ethanone. Int J Pharm Sci Res 4:713–717
Vinayak A, Sudha M, Jagadeesha AH, Lalita KS, Rao K (2014) Synthesis, characterization and cytotoxic evaluation of novel derivatives of 1, 3, 4-oxadiazole containing 5-phenyl thiophene moiety. IOSR- JPBS 9:42–48
Vinayak A, Sudha M, Rao K, Lalita KS (2013) Synthesis, characterisation and anticancer acitivity of schiff base derivatives of 5-(2-phenoxypyridin-3-yl)-1, 3, 4-thiadiazol-2-amine. Int Res J Pharm 4:62–66
Vinayak A, Sudha M, Rao K, Lalita KS (2014) synthesis of n-{[5-(2,4-dichlorophenyl)- 1, 3, 4-oxadiazol- 2-yl] methyl} amine derivatives as anticancer precursors. Int J Med Chem Anal 4:231–235
Vinayak A, Anusha S, Santosh N, Basappa Y (2020) Synthesis, impedance, and current–voltage characteristics of strontium-manganese titanate hybrid nanoparticles. Macromol Symp 392:2000002
Vinayak A, Revaigh MG, Adarsha HJ (2020) Synthesis and fabrication of y-doped ZnO nanoparticles and their application as a gas sensor for the detection of ammonia. J Mater Eng Perform 29:4–5
Wang B, Gu Y, Luo C, Yang T, Yang L, Suo J (2004) Pyrrole synthesis in ionic liquids by Paal-Knorr condensation under mild conditions. Tetrahedron Lett 45:3417–3419
Li X, Chen M, Xie X, Sun N, Li S, Liu Y (2015) Synthesis of multiple-substituted pyrroles via gold (I)-catalyzed hydroamination/cyclization cascade. Organic Lett 17:2984–2987
Yamamoto H, Sasaki I, Mitsutake M, Karasudani A, Imagawa H, Nishizawa M (2011) An efficient pyrrole synthesis via silaphenylmercuric triflate catalyzed cyclization of homopropargyl azides. Synlett 2011:2815–2818
Kim Y, Kim J, Park SB (2009) Regioselective synthesis of tetrasubstituted pyrroles by 1, 3-dipolar cycloaddition and spontaneous decarboxylation. Organic Lett 11:17–20
St Cyr DJ, Arndtsen BA (2007) A new use of Wittig-type reagents as 1, 3-dipolar cycloaddition precursors and in pyrrole synthesis. J Am Chem Soc 129:12366–12367
Bonnaud B, Bigg DC (1994) Preparation of pyrroles by dehydrogenation of pyrrolidines with manganese dioxide. Synthesis 1994:465–467
Veltri L, Mancuso R, Altomare A, Gabriele B (2015) Divergent multicomponent tandem palladium-catalyzed aminocarbonylation-cyclization approaches to functionalized imidazothiazinones and imidazothiazoles. ChemCatChem 7:2206–2213
Cioc RC, Ruijter E, Orru RV (2014) Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem 16:2958–2975
Das D (2016) Multicomponent reactions in organic synthesis using copper-based nanocatalysts. ChemistrySelect 1:1959–1980
van der Heijden G, Ruijter E, Orru RV (2013) Efficiency, diversity, and complexity with multicomponent reactions. Synlett 24:666–685
Marson CM (2012) Multicomponent and sequential organocatalytic reactions: diversity with atom-economy and enantiocontrol. Chem Soc Rev 41:7712–7722
Rahman I, Deka B, Thakuria R, Deb ML, Baruah PK (2020) l-Proline-catalyzed regioselective C1 arylation of tetrahydroisoquinolines through a multicomponent reaction under solvent-free conditions. Org Biomol Chem 18:6514–6518
Dandapani S, Marcaurelle LA (2010) Current strategies for diversity-oriented synthesis. Curr Opin Chem Biol 14:362–370
Dutta A, Patra SK, Khatua S, Nongkhlaw R (2021) Visible-light-mediated synthesis of 3, 4, 5-trisubstituted furan-2-one derivative via bifunctional based organo photocatalyst. New J Chem. https://doi.org/10.1039/D1NJ03238K
Zhang M, Chen MN, Li JM, Liu N, Zhang ZH (2019) Visible-light-initiated one-pot, three-component synthesis of 2-amino-4 h-pyran-3, 5-dicarbonitrile derivatives. ACS Combinatorial Sci 21:685–691
Chen J, Liu S, Lv X, Hong K, Lei J, Xu X, Hu W (2020) Blue light-promoted formal [4+ 1]-annulation of diazoacetates with o-aminoacetophenones: synthesis of polysubstituted indolines and computational study. J Org Chem 85:13920–13928
Jarrahi M, Tayebee R, Maleki B, Salimi A (2021) One-pot multicomponent green LED photoinduced synthesis of chromeno [4, 3-b] chromenes catalyzed by a new nanophotocatalyst histaminium tetrachlorozincate. RSC Adv 11:19723–19736
Harale RR, Shitre PV, Sathe BR, Shingare MS (2017) Visible light motivated synthesis of polyhydroquinoline derivatives using CdS nanowires. Res Chem Intermed 43:3237–3249
Cai BG, Li Q, Zhang Q, Li L, Xuan J (2021) Synthesis of trisubstituted hydroxylamines by visible light-promoted multicomponent reaction. Org Chem Front. https://doi.org/10.1039/D1QO01102B
Zhu J, Dai C, Ma M, Yue Y, Fan X (2021) Visible light-mediated cross-coupling of electrophiles: synthesis of α-amino amides from isocyanates and ketimines. Org Chem Front 8:1227–1232
Wang X, Zhu B, Dong J, Tian H, Liu Y, Song H, Wang Q (2021) Visible-light-mediated multicomponent reaction for secondary amine synthesis. Chem Commun 57:5028–5031
Wu CJ, Meng QY, Lei T, Zhong JJ, Liu WQ, Zhao LM, Li ZJ, Chen B, Tung CH, Wu LZ (2016) An oxidant-free strategy for indole synthesis via intramolecular C-C bond construction under visible light irradiation: cross-coupling hydrogen evolution reaction. ACS Catal 6:4635–4639
Patel G, Patel AR, Banerjee S (2020) Visible light-emitting diode light-driven one-pot four component synthesis of poly-functionalized imidazoles under catalyst-and solvent-free conditions. New J Chem 44:13295–13300
Tabassum S, Govindaraju S, Pasha MA (2016) Ultrasound mediated, green innovation for the synthesis of polysubstituted 1, 4-dihydropyridines. RSC Adv 6:29802–29810
Tabassum S, Govindaraju S, Pasha MA (2017) FeSO4⋅7H2O catalyzed rapid and efficient one-pot multicomponent synthesis of functionalized pyrazol-yl-pyrazolone methanes. ChemistrySelect 2:4054–4057
Acknowledgements
The authors thank SIF, IISc Bangalore for spectral analysis.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Govindaraju, S., Tabassum, S. Visible Light Mediated Organophotoredox-Catalyzed One-Pot Domino Synthesis of Novel 6,7 Disubstituted 1H-Pyrroles. Top Catal (2022). https://doi.org/10.1007/s11244-022-01580-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11244-022-01580-y