Abstract
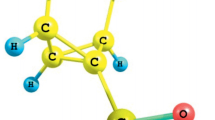
Establishing of the structure of hydrazinolysis product obtained from bicyclo[2.2.1]hept-2-ene-endo-5, endo-6-dicarboxylic (endic) acid was performed by preparation of the compound under alternative conditions followed by comparison of the characteristics and spectral parameters of the resulting substances, and also by quantum-chemical calculations by the density functional method of the chemical shifts in 1H and 13C NMR spectra of different reaction products. The X-ray diffraction analysis of the hydrazide was also carried out. The compound obtained was assigned a structure of N-aminobicyclo[2.2.1] hept-2-ene-endo-5,endo-6-dicarboximide. The products were prepared by its reactions with arylsulfonyl chlorides, benzoyl chlorides, m-tolyl and p-toluene-sulfonyl isocyanates, phenyl isothiocyanate, with o-nitrobenzaldehyde, and oxiranes (1,2-epoxycyclohexane and 2,3-epoxypropylcarbazole). The aromatic sulfonamides, carboxamides, and ureas were epoxidized by performic acid obtained in situ from the formic acid and hydrogen peroxide. Products of [3+2]-cycloaddition of aryl azides to the strained double bond in the N-aminobicyclo[2.2.1] hept-2-ene-endo-5,endo-6-dicarboximide and its derivatives. The structures of compounds obtained were confirmed by their IR, 1H and 13C NMR spectra.
Similar content being viewed by others
REFERENCES
Grekov, A.P., Organicheskaya khimiya gidrazina (Organic Chemistry of Hydrazine), Kiev: Tekhn3ka, 1966, 235 p.; Overberg, Ch.J., Anselm, G.-P., and Lombardino, G.G., Organicheskie coedineniya so svyazyami azot-azot (Organic Compounds with Nitrogen-Nitrogen Bonds), Leningrad: Khimiya, 1970, 123 p.; Shvaika, Ol., Osnovi sintezu likars'kikh rechovin (Principles of Synthesis of Medicines), Donets'k: Skhidnii Vidavnichii Dim, 2002, 304 p.
US Patent 3277111, 1966; Ref. Zh. Khim., 1968, 6N729P.
Japan Patent 9319, 1967; Ref. Zh. Khim., 1968, 21N595P; Furdik, M., Sidoova, E., and Priechrandy, S., Acta Fac. Rerum. Nature. Univ. Com. Chim., 1968, 12, 253; Japan Patent 59-122465, 1984; Ref. Zh. Khim., 1985, 15O39P; Koval, I., Sulfur Reports, 1993, vol. 14, p. 149.
Krishchik, O.V., Tarabara, I.N., Kas'yan, A.O., Shishkina, S.V., Shishkin, O.V., Isaev, A.K., and Kas'yan, L.I., Zh. Org. Khim., 2004, vol. 40, p. 1188.
Japan Patent 48-2552, (1972); Ref. Zh. Khim., 1973, 23N365P.
Csende, F. and Czabo, Z., Synth. Commun., 1993, vol. 23, p. 2957.
Augustin, M. and Reinemann, P., Z. Chem., 1972, vol. 12, p. 101.
Augustin, M. and Reinemann, P., Z. Chem., 1973, vol. 13, p. 61.
Auksi, H. and Yates, P., Can. J. Chem., 1981, vol. 59, p. 2510.
Hedaya, E. and Hinman, R.L., J. Org. Chem., 1966, vol. 31, p. 1317.
Essawy, S.A., El-Kady, M.V., Metwelly, R.N., and El-Shenawy, A.J., 4th Ibn Sina Int. Symp. Pure and Appl. Heterocycl. Chem., Cairo, 1992, p. 32.
Nakanisi, K., Infrakrasnye spektry i stroenie organicheskikh soedinenii (IR Spectra and Structure of Organic Compounds), Moscow: Mir, 1965, 209 p.; Bellamy, L.J., The Infra-red Spectra of Complex Molecules, London: Methuen, 1958.
Zefirov, N.S. and Sokolov, V.I., Usp. Khim., 1967, vol. 36, p. 243.
Ditchfield, R., Chem. Phys. Lett., 1972, 15, 203; Wolinksi, K., Hinton, J.F., and Pulay, P.J., J. Am. Chem. Soc., 1990, vol. 112, p. 8251; Okovityi, S.I., Bol'shakov, V.I., Voronkov, E.O., Rossikhin, V.V., and Umrykhina, L.K., Novin nauki Pridniprov'ya, 2004, vol. 5, p. 20.
Burgi, H.-B. and Dunitz, J.D., Structure Correlation, VCH, 1994, vol. 2, p. 741.
Davies, R.G., Gibson, V.C., Hursthouse, M.B., Light, M.E., Marshall, E.L., North, M., Robson, D.A., Thompson, I., White, A.J.P., Williams, D.J., and Williams, P.J., J. Chem. Soc., Perkin Trans. I, 2001, p. 3365; Min, J., Benet-Buchholz, J., and Boese, R., Chem. Commun., 1998, p. 2715; Bolte, M., Degen, A., and Egert, E., Acta Crystallogr., 2000, vol. 56, p. 1338; Pashnova, L.V., Svetlichnaya, V.M., Kudryavtseva, N.E., Stash, F.I., Bel'skii, V.K., and Skvortsova, N.K., Zh. Obshch. Khim., 1995, vol. 65, p. 1968.
Kas'yan, L.I., Okovityi, S.I., and Kas'yan, A.O., Zh. Org. Khim., 2004, vol. 40, p. 11.
Kirk, D.N., Chem. Ind., 1973, p. 109.
Kas'yan, L.I., Kas'yan, A.O., Okovityi, S.I., and Tarabara, I.N., Alitsiklicheskie epoxydnye soedineniya. Reaktsionnaya sposobnost' (Alicyclic Epoxide Comounds. Reactivity), Dnepropetrovsk, 2003, 510 p.
Dryuk, V.G., Kartsev, V.G., and Voitsekhovskaya, M.A., Oksirany-sintez i biologicheskaya aktivnost' (Oxiranes: Synthesis and Biological Activity), Moscow: Bogorodskii Pechatnik, 1999, 527 p.
Prilezhaeva, E.N., Reaktsiya Prilezhaeva. Elektrofil'noe okislenie (Reaction of Prolezhaev. Electrophilic Oxidation), Moscow: Nauka, 1974, 332 p.
Kas'yan, L.I., Usp. Khim., 1998, vol. 67, p. 299; Kas'yan, L.I., Zh. Org. Khim., 1999, vol. 35, p. 661; Kas'yan, L.I., Seferova, M.F., and Okovityi, S.I., Alitsiklicheskie epoxydnye soedineniya. Metody sinteza (Alicyclic Epoxide Compounds. Methods of Synthesis), Dnepropetrovsk, 1996, 192 p.
Tori, K., Aono, K., Kitahonoki, K., Muneyuki, R., Takano, Y., Tanida, H., and Tsuji, T., Tetrahedron Lett., 1966, p. 2921; Zefirov, N.S., Kasyan, L.I., Gnedenkov, L.Yu., Shashkov, A.S., and Cherepanova, E.G., Tetrahedron Lett., 1979, p. 949.
L'abbe, G., Chem. Rew., 1969, vol. 69, p. 345; Scriven, E.F.V. and Turnbull, K., Chem. Rew., 1988, vol. 88, p. 297; Shea, K.J. and Kim, J., J. Am. Chem. Soc., 1992, vol. 114, p. 4846; Scheiner, P., Schomaker, J.H., and Deming, S., J. Am. Chem. Soc., 1965, vol. 87, p. 306.
Kas'yan, L.I., Tarabara, I.N., Kas'yan, A.O., and Yarovoi, M.Yu., Zh. Org. Khim., 2003, vol. 39, p. 1698; Tarabara, I.N., Kas'yan, A.O., Yarovoi, M.Yu., Shishkina, S.V., Shishkin, O.V., and Kas'yan, L.I., Zh. Org. Khim., 2004, vol. 40, p. 1033.
Sheldrick, G.M., SHELXTL PLUS PC, Version. A system of Computer Programs for the Determination of Crystal Structure from X-ray Diffraction data, 1998.
Author information
Authors and Affiliations
Additional information
__________
Translated from Zhurnal Organicheskoi Khimii, Vol. 41, No. 8, 2005, pp. 1145–1154.
Original Russian Text Copyright © 2005 by Kas'yan, Tarabara, Bondarenko, Shishkina, Shishkin, Musatov.
Rights and permissions
About this article
Cite this article
Kas'yan, L., Tarabara, I., Bondarenko, Y. et al. Structure and Reactivity of Bicyclo[2.2.1]hept-2-ene-endo-5,endo-6-dicarboxylic (endic) Acid Hydrazide. Russ J Org Chem 41, 1122–1131 (2005). https://doi.org/10.1007/s11178-005-0305-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11178-005-0305-9