Abstract
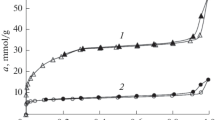
Methane adsorption by the sample of the metal-organic framework structure (MOF) Zr-BDC (BDC is benzene-1,4-dicarboxylate) containing zirconium ions was studied in a pressure range of 0.1–40 MPa at 303, 313, 323, and 333 K. The excess adsorption isotherms Γ(P,T) and adsorption isotherms of total amount a(P, T) were calculated, and the adsorption volume W was determined. The adsorption isosteres were constructed on the basis of these isotherms, and the isosteric qst(a,T) and initial q(0,T) heats of adsorption were calculated. The average isosteric heats of adsorption q *st (a, T) were determined taking into account the dependence of qst(a,T) on the amount adsorbed. These values were compared with the average heats of adsorption q(E) calculated with allowance for the characteristic energy of adsorption E(T), which were determined, in turn, from the adsorption isotherms a(P,T). Two calculation methods were used: via the characteristic curve and via the adsorption isotherm presented in the linear coordinates of the Dubinin—Astakhov equation. In both cases, new parameters were introduced in the adsorption equation to determine E(T): “Ps” that replaces the vapor pressure at saturation and critical temperature Tc. The Zr-BDC MOF sample is a microporous adsorbent, which can be used for methane storage.
Similar content being viewed by others
References
V. V. Butova, M. A. Soldatov, A. A. Guda, R. A. Lomatshenko, C. Lamberti, Russ. Chem. Rev., 2016, 85, 280.
A. Yu. Tsivadze, O. E. Aksyutin, A. G. Ishkov, M. K. Knyazeva, O. V. Solovtsova, I. E. Men’shchikov, A. A. Fomkin, A. V. Shkolin, E. V. Khozina, V. A. Grachev, Russ. Chem. Rev., 2019, 88, 923.
A. Yu. Tsivadze, O. E. Aksyutin, A. G. Ishkov, I. E. Men’shchikov, A. A. Fomkin, A. V. Shkolin, E. V. Khozina, V. A. Grachev, Russ. Chem. Rev., 2018, 87, 950.
A. A. Fomkin, A. A. Pribylov, A. V. Shkolin, I. E. Men’shchikov, K. O. Murdmaa, A. L. Pulin, Russ. Chem. Bull., 2019, 68, 1838.
M. Rezakazemi, M. Sadazedek, T. Matsuura, Progr. Energy Combust Sci., 2018, 66, 1.
P. M. Schoenecher, C. G. Carson, H. Jasuja, C. J. J. Flemming, K. S. Walton, Ind. Eng. Chem. Res., 2012, 51, 6513.
H. R. Abid, H. Tian, H. M. Ang, M. O. Tade, C. E. Buckley, S. Wang, Chem. Eng. J., 2012, 187, 415.
A. D. Wiersum, J.-S. Chang, C. Serre, P. L. Liewellun, Langmuir, 2013, 29, 3301.
J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850.
S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li, H.-C. Zhou, Adv. Mater., 2018, 30, 1704303.
A. A. Pribylov, S. M. Kalashnikov, V. V. Serpinskii, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1990, 39, 1115.
V. V. Sychev, A. A. Vasserman, V. A. Zagoruchenko, A. D. Kozlov, G. A. Spiridonov, V. A. Tsymarnyi, Termodinamicheskie svoistva metana [Thermodynamic Properties of Methane], Izd-vo Standartov, Moscow, 1979, 348 pp. (in Russian).
T. S. Yakubov, L. G. Shekhovtsova, A. A. Pribylov, Russ. Chem. Bull., 1995, 44, 2277.
V. A. Bakaev, Dokl. AN SSSR, 1966, 167, 369 [Dokl. Acad. Sci. USSR (Engl. Transl.), 1966, 167].
M. M. Dubinin, Adsorbtsiya i poristost’ [Adsorption and Porosity], Izd-vo VAKhZ, Moscow, 1972, 127 pp. (in Russian).
A. A. Pribylov, K. O. Murdmaa, Russ. Chem. Bull., 2017, 66, 849.
B. P. Bering, E. G. Žukovskaja, B. Kh. Rakhmukov, V. V. Serpinskii, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1967, 16, 1597.
B. P. Bering, E. G. Žukovskaja, B. Kh. Rakhmukov, V. V. Serpinskii, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1967, 16, 1603.
B. P. Bering, E. G. Žukovskaja, B. Kh. Rakhmukov, V. V. Serpinskii, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1968, 17, 11.
A. A. Pribylov, K. O. Murdmaa, O. V. Solovtsova, M. K. Knyazeva, Russ. Chem. Bull., 2018, 67, 1807.
A. A. Pribylov, K. O. Murdmaa, Russ. Chem. Bull., 2019, 68, 2002.
A. A. Pribylov, I. A. Kalinnikova, K. O. Murdmaa, Russ. Chem. Bull., 2016, 65, 972.
M. M. Dubinin, V. A. Astakhov, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1971, 20, 3.
K. M. Nikolaev, M. M. Dubinin, Bull. Acad. Sci. USSR, Div. Chem., 1958, 7, 1124.
M. M. Dubinin, Russ. Chem. Rev., 1982, 51, 605.
O. V. Solovtsova, A. L. Pulin, I. E. Men’shchikov, N. P. Platonova, M. K. Knyazeva, S. S. Tchugaev, A. V. Shkolin, A. A. Fomkin, Fizikokhim. Poverkhn. Zashchita Mater., 2020, 56, No. 6, 8 [Protect. Met. Phys. Chem. Surf. (Engl. Transl.), 2020, 56, No. 6].
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 665–671, April, 2021.
This work was carried out in terms of state assignment No. 1201353185.
This work does not involve human participants and animal subjects.
The authors declare that there is no conflict of interest in financial or any other sphere.
Rights and permissions
About this article
Cite this article
Pribylov, A.A., Murdmaa, K.O. & Solovtsova, O.V. Methane adsorption on the Zr-BDC metal-organic framework structure at supercritical temperatures and pressures. Russ Chem Bull 70, 665–671 (2021). https://doi.org/10.1007/s11172-021-3134-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3134-2