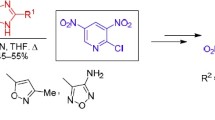
For the first time pyrazole containing three nitro groups in the pyrazole cycle, viz., 1,3,4-tri-nitropyrazole, was obtained by N-nitration of 3,4-dinitropyrazole. 1,3,4-Trinitropyrazole enters the cine-substitution reaction of N-nitro group under the action of 0-, N-, and C-nucleophyls under mild conditions. Thus, general method of synthesis of 5-substituted 3,4-dinitropyrazoles containing free NH fragment was worked out.
Similar content being viewed by others
References
M. I. Kanishchev, N. V. Korneeva, S. A. Shevelev, A. A. Fainzilberg, Khim. Geterotsikl. Soedin., 1988, 435 [Chem. Heterocycl. Compd. (Engl. Transl.), 1988, 24, 353].
I. L. Dalinger, S. A. Shevelev, Zh. Org. Khim., 1998, 34, 1127 [Russ. J. Org. Chem. (Engl. Transl.), 1998, 34, 1071].
V. P. Perevalov, M. A. Andreeva, L. I. Baryshnenkova, Yu. A. Minaev, E. S. Yamburg, B. I. Stepanov, V. A. Dubrovskaya, Khim. Geterotsikl. Soedin., 1983, 1676 [Chem. Heterocycl. Compd. (Engl. Transl.), 1983, 19, 1326].
Ch. L. Habraken, E. K. Poels, J. Org. Chem., 1977, 42, 2893.
P. Cohen-Fernandes, C. Erkelens, C. Endenburg, J. J. Verkoeven, Ch. L. Habraken, J. Org. Chem., 1979, 44, 4156.
R. Berbee, Ch. L. Habraken, J. Heterocycl. Chem., 1981, 18, 559.
J. G. Buchanan, A. Stobie, R. H. Wightman, Can. J. Chem., 1980, 58, 2624.
J. G. Buchanan, D. Smith, R. H. Wightman, Tetrahedron, 1984, 40, 119.
J. G. Buchanan, D. Smith, R. H. Wightman, J. Chem. Soc., Perkin Trans. 1, 1986, 1267.
J. G. Buchanan, M. Harrison, R. H. Wightman, M. R. Hardner, J. Chem. Soc., Perkin Trans. 1, 1989, 925.
J. G. Buchanan, A. O. Jumaah, G. Kerr, R. R. Talekar, J. Chem. Soc., Perkin Trans. 1, 1991, 1077.
J. G. Buchanan, A. R. Edgar, R. J. Hutchison, A. Stobie, R. H. Wightman, J. Chem. Soc., Perkin Trans. 1, 1980, 2567; J. G. Buchanan, A. Stobie, R. H. Wightman, J. Chem. Soc., Perkin Trans. 1, 1981, 2374; J. G. Buchanan, N. K. Saxena, R. H. Wightman, J. Chem. Soc., Perkin Trans. 1, 1984, 2367.
Author information
Authors and Affiliations
Corresponding author
Additional information
On the occasion of the 75th anniversary of the foundation of the N. D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 2, pp. 404–406, February, 2009.
Rights and permissions
About this article
Cite this article
Dalinger, I.L., Cherkasova, T.I., Popova, G.P. et al. Nitropyrazoles 14. Synthesis of 1,3,4-trinitropyrazole and its behavior in the nucleophilic substitution reactions. General method of synthesis of 5-substituted 3,4-dinitropyrazoles. Russ Chem Bull 58, 410–413 (2009). https://doi.org/10.1007/s11172-010-0024-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-010-0024-4