Abstract
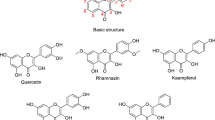
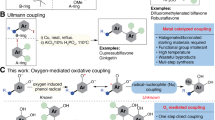
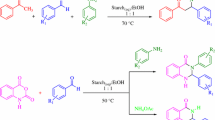
Herein, alkaline amylopectin (AAp) was prepared under chemo-mechanically dry conditions, characterized by base capacity, FT-IR, FESEM, TEM, and TGA analyses, and held as a new bio-organic base catalyst in the one-pot synthesis of flavonols. With a base capacity of 7.3 mmolHO−/g, AAp catalyzed initial aldol condensation and further Algar–Flynn–Oyamada reaction in cooperation with hydrogen peroxide to give a high yield of flavonol antioxidants. So, the first aldol condensation or the latter oxidative cyclization/hydroxylation of the in situ-formed 2´-hydroxy-chalcones proceeded successfully in a pot to form flavonol products without aurone side product. The structure of the supra-molecular base catalyst AAp was found to be well preserved after the five times reusing. The rewards of this protocol are available reactants, no aurone side product, short reaction time, high yield, and catalyst reusability.
Graphical abstract










Similar content being viewed by others
Availability of data and materials
The data that support outcomes of this study are available on request from the corresponding author.
References
B. Madan, S. Batra, B. Ghosh, Mol. Pharmacol. 58, 3 (2000)
K. Maria, H.-L. Dimitra, G. Maria, Med. Chem. 4, 6 (2008)
B.K. Singh, V. Kumar, S. Malhotra, M.K. Pandey, R. Jain, R. Thimmulappa, S.K. Sharma, A.K. Prasad, S. Biswal, E. Van der Eycken, Arch. Pharm. Chem 345, 1 (2012)
J. Vaya, P.A. Belinky, M. Aviram, Free Radical Biol. Med. 23, 2 (1997)
A. Wilhelm, S.L. Bonnet, L. Twigge, L. Rarova, T. Stenclova, H.G. Visser, M. Schutte-Smith, J. Mol. Struct. 1251, 1 (2022)
A. Raval, N. Shah, J. Org. Chem. 23, 5 (1958)
S.S. Choudhury, S. Mahapatra, A.K. Sahu, P. Hembram, S. Jena, H.S. Biswal, A.C.S. Sustain, Chem. Eng. 10, 43 (2022)
A.K. Verma, B.N. Kishor, O. Prakash, Mini Rev. Org. Chem. 19, 6 (2022)
A. Rammohan, J.S. Reddy, G. Sravya, C.N. Rao, G.V. Zyryanov, Environ. Chem. Lett. 18, 2 (2020)
J. Zhu, B. Xu, J. Yu, Y. Ren, J. Wang, P. Xie, C.U. Pittman, A. Zhou, Org. Biomol. Chem. 16, 33 (2018)
S.K. Jash, G. Brahmachari, Org. Biomol. Chem. 1, 1 (2013)
W. Alam, H. Khan, M.A. Shah, O. Cauli, L. Saso, Molecules 25, 18 (2020)
R.G. Britton, E. Horner-Glister, O.A. Pomenya, E.E. Smith, R. Denton, P.R. Jenkins, W.P. Steward, K. Brown, A. Gescher, S. Sale, Eur. J. Med. Chem. 54, 1 (2012)
S. Cai, Y. Kong, D. Xiao, Y. Chen, Q. Wang, Org. Biomol. Chem. 16, 11 (2018)
S. Prafulla, P. Lata, S. Nusrat, R. Priya, Syst. Rev. Pharm. 12, 12 (2021)
Z. Yazdiniapour, A. Yegdaneh, S. Akbari, Adv. Biomed. Res. 10, 1 (2021)
J.A. Seijas, M.P. Vázquez-Tato, R. Carballido-Reboredo, J. Org. Chem. 70, 7 (2005)
S. Bhagwat, D.B. Haytowitz and J.M. Holden, USDA database for the flavonoid content of selected foods, Release 3.1, (Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA, 2014)
S. Vimalraj, S. Saravanan, G. Hariprabu, R. Yuvashree, S.K.A. Kanna, K. Sujoy and D. Anjali, Life Sci. 256, (2020)
P. Rajendran, T. Rengarajan, N. Nandakumar, R. Palaniswami, Y. Nishigaki, I. Nishigaki, Eur. J. Med. Chem. 86, 1 (2014)
M.a.T. Mercader-Ros, C. Lucas-Abellan, J.A. Gabaldón, M.a.I. Fortea, A. Martinez-Cacha and E. Nunez-Delicado, Journal of agricultural and food chemistry 58, 8 (2010)
D. Kashyap, A. Sharma, H.S. Tuli, K. Sak, S. Punia, T.K. Mukherjee, J. Funct. Foods 30, 1 (2017)
L. Duan, W. Ding, X. Liu, X. Cheng, J. Cai, E. Hua, H. Jiang, Microb. Cell Fact. 16, 1 (2017)
J.M. Calderon-Montano, E. Burgos-Morón, C. Pérez-Guerrero, M. López-Lázaro, Mini Rev. Med. Chem. 11, 4 (2011)
J. Zhang, W. Xiong, Y. Wen, X. Fu, X. Lu, G. Zhang, C. Wang, Org. Biomol. Chem. 20, 5 (2022)
S. Paul, A.K. Bhattacharya, Org. Biomol. Chem. 16, 3 (2018)
A. St-Gelais, J. Alsarraf, J. Legault, C. Gauthier, A. Pichette, Org. Lett. 20, 23 (2018)
J.J. Li, in Name Reactions(Springer, 2003), pp. 13–13
J. Algar and J.P. Flynn, in Proc. R. Ir. Acad. B(JSTOR, 1934), pp. 1–8
T. Oyamada, J. Chem. Soc. Jpn 55, 1 (1934)
T. Oyamada, Bull. Chem. Soc. Jpn. 10, 5 (1935)
B. Cummins, D.M.X. Donnelly, J.F. Eades, H. Fletcher, F.O. Cinnéide, E.M. Philbin, J. Swirski, T.S. Wheeler, R.K. Wilson, Tetrahedron 19, 4 (1963)
W. Xiong, X. Wang, X. Shen, C. Hu, X. Wang, F. Wang, G. Zhang, C. Wang, J. Org. Chem. 85, 20 (2020)
D. Donnelly, J. Eades, E. Philbin and T. Wheeler, (Soc Chemical Industry 14 BELGRAVE SQUARE, LONDON SW1X 8PS, ENGLAND, 1961), pp. 1453–1453
T. Geissman, D.K. Fukushima, J. Am. Chem. Soc. 70, 5 (1948)
S. Bhattacharyya, K. Hatua, RSC Adv. 4, 36 (2014)
C. Espíndola, Mini Rev. Org. Chem. 17, 6 (2020)
T. Roy, S.T. Boateng, S. Banang-Mbeumi, P.K. Singh, P. Basnet, R.-C.N. Chamcheu, F. Ladu, I. Chauvin, V.S. Spiegelman, R.A. Hill, Bioorg. Chem. 107, 1 (2021)
A. Kumar, S. Kumar, J.K. Makrandi, Green Process. Synth. 2, 4 (2013)
U.C. Rajesh, S. Manohar, D.S. Rawat, Adv. Synth. Catal. 355, 16 (2013)
S. Kumar, R. Ambatwar, V. Gupta, G.L. Khatik, Res. Chem. Intermed. 49, 3 (2023)
X. Shen, Q. Zhou, W. Xiong, W. Pu, W. Zhang, G. Zhang, C. Wang, Tetrahedron 73, 32 (2017)
R.W. Welford, I.J. Clifton, J.J. Turnbull, S.C. Wilson, C.J. Schofield, Org. Biomol. Chem. 3, 17 (2005)
M.M. Cox, Lehninger principles of biochemistry (2013)
R. Mukerjea, J.F. Robyt, Carbohydr. Res. 345, 3 (2010)
D. Le Corre, J. Bras, A. Dufresne, Biomacromol 11, 5 (2010)
S. Naguleswaran, T. Vasanthan, R. Hoover, D. Bressler, Food Hydrocoll. 35, 1 (2014)
K. Alvani, X. Qi, R.F. Tester, C.E. Snape, Food Chem. 125, 3 (2011)
S.-Z. Zhang, Z.-S. Cui, M. Zhang, Z.-H. Zhang, Curr. Opin. Green Sustain. Chem. 38, 1 (2022)
L.-N. Dong, S.-Z. Zhang, W.-L. Zhang, Y. Dong, L.-P. Mo, Z.-H. Zhang, Res. Chem. Intermed. 48, 3 (2022)
F.G. Torres, G.E. De-la-Torre, Int. J. Biol. Macromol. 194, 1 (2022)
M.C. Sweedman, M.J. Tizzotti, C. Schäfer, R.G. Gilbert, Carbohydr. Polym. 92, 1 (2013)
N. Masina, Y.E. Choonara, P. Kumar, L.C. du Toit, M. Govender, S. Indermun, V. Pillay, Carbohydr. Polym. 157, 1 (2017)
Y. Zhou, H. Essawy, A. Liu, C. Yang, D. Hou, X. Zhou, G. Du, J. Zhang, J. Renew. Mater. 11, 2 (2023)
F. Tamaddon, M. KazemiVarnamkhasti, Carbohydr. Res. 437, 1 (2017)
F. Tamaddon and M. Taghi Kazemi Varnamkhasti, Curr. Catal. 6, 1 (2017)
F. Tamaddon, H. Rashidi, Res. Chem. Intermed. 49, 8 (2023)
S. Kiran, P. Rohini, P. Bhagyasree, Journal of Pharmacognosy and Phytochemistry 6, 5 (2017)
K. Yamasaki, R. Hishiki, E. Kato, J. Kawabata, A.C.S. Med, Chem. Lett. 2, 1 (2011)
S. Bhagwat, D.B. Haytowitz, J.M. Holden, US Department of Agriculture: Beltsville (MD, USA, 2014)
R.A. Sheldon, M. Wallau, I. Arends, U. Schuchardt, Acc. Chem. Res. 31, 8 (1998)
F. Tamaddon, F. Pouramini, Synlett 25, 08 (2014)
F. Tamaddon, E. Ahmadi-AhmadAbadi, H. Kargar, Appl. Organomet. Chem. 35, 11 (2021)
M. Babu, K. Pitchumani, P. Ramesh, Helv. Chim. Acta 96, 7 (2013)
A. Karmakar, P. Ambure, T. Mallick, S. Das, K. Roy, N.A. Begum, Med. Chem. Res. 28, 1 (2019)
A.M. Sobottka, W. Werner, G. Blaschke, W. Kiefer, U. Nowe, G. Dannhardt, E.E. Schapoval, E.P. Schenkel, G.K. Scriba, Arch. Pharm. 333, 7 (2000)
E. Venkateswararao, M.-J. Son, N. Sharma, M. Manickam, P. Boggu, Y.H. Kim, S.-H. Woo, S.-H. Jung, A.C.S. Med, Chem. Lett. 6, 7 (2015)
P. Kamble, S. Wadher, Synthesis 11, 3 (2018)
M.-L. Ma, M. Li, J.-J. Gou, T.-Y. Ruan, H.-S. Jin, L.-H. Zhang, L.-C. Wu, X.-Y. Li, Y.-H. Hu, K. Wen, Biorg. Med. Chem. 22, 21 (2014)
M. Parveen, A. Aslam, S. Siddiqui, M. Tabish, M. Alam, J. Mol. Struct. 1251, 1 (2022)
Acknowledgements
The authors appreciate the Yazd University Research Council.
Funding
With a fractional support by the Yazd University, this work is fully related to the PhD dissertation of Hossein Rashidi under supervision of Prof. Fatemeh Tamaddon at Organic Division of Chemistry Department of Yazd University.
Author information
Authors and Affiliations
Contributions
FT (corresponding author) did conceptualization, supervision, validation, project administration, software, formal analysis, and visualization. HR done methodology and investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
All authors declare that they have no known conflict of interest, competing financial interest, or personal relationship that could have appeared to influence the work reported in this paper.
Ethical approval
No human and/ or animal trials have been examined in this project.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tamaddon, F., Rashidi, H. Green access to flavonols by one-pot serial aldol condensation/Algar–Flynn–Oyamada reaction catalyzed using the new bio-based catalyst of alkaline amylopectin. Res Chem Intermed 49, 5221–5236 (2023). https://doi.org/10.1007/s11164-023-05138-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05138-9