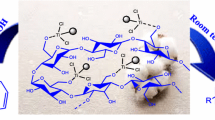
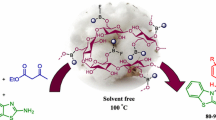
Abstract
A novel nanomagnetic catalyst has been synthesized by immobilization of triethanolamine lactate cellulose with Fe3O4 NPs. The prepared catalyst has been used as an efficient and recoverable catalyst for the regioselective synthesis of pyrazolo quinolones by the three-component reaction of dimedone, 5-amino pyrazolone and aromatic aldehydes in ultrasound condition. This economical catalyst provides high yields of products, short experimental time and easy workup for green synthesis of pyrazolo[3,4-b]quinolone compounds. Theoretical studies have been employed to investigate the reaction mechanism by using the B3LYP/6-311G method. Electronic parameters of all components have been determined in order to reorganize the role of components in the reaction mechanism.
Graphic abstract















Similar content being viewed by others
References
X.-L. Sun, J.C. Zheng, Y. Tang, Pure Appl. Chem. 82, 625 (2010)
W.R. Vaughan, Chem. Rev. 43, 447 (1948)
H.W. Heine, R. Henrie, L. Heitz, S.R. Kovvali, J. Org. Chem. 39, 3187 (1974)
T. Sheradsky, R. Moshenberg, J. Org. Chem. 51, 3123 (1986)
C. Turk, J. Svete, B. Stanovnik, L. Golič, S. Golič-Grdadolnik, A. Golobič, L. Selič, Helv. Chim. Acta 84, 146 (2001)
G. Heinisch, H. Frank, in Progress in Medicinal Chemistry, ed. by G.P. Ellis and G.B. West (Elsevier, Amsterdam, 1990), pp. 1–49
M. Zahedifar, B. Pouramiri, F. Ezzati Ghadi, R. Razavi, A. Ramzani Ghara, Mol. Divers. (2019). https://doi.org/10.1007/s11030-019-10022-z
A.H. El-Wahab, Z.I. Al-Fifi, A.H. Bedair, F.M. Ali, A.H. Halawa, A.M. El-Agrody, Molecules 16, 307 (2011)
G. Ouyang, X.-J. Cai, Z. Chen, B.-A. Song, P.S. Bhadury, S. Yang, L.-H. Jin, W. Xue, D.-Y. Hu, S. Zeng, J. Agric. Food. Chem. 56, 10160 (2008)
H.A. Saad, N.A. Osman, A.H. Moustafa, Molecules 16, 10187 (2011)
H. Bienaymé, C. Hulme, G. Oddon, P. Schmitt, Chem. Eur. J. 6, 3321 (2000)
A. Nefzi, J.M. Ostresh, R.A. Houghten, Chem. Rev. 97, 449 (1997)
L.A. Thompson, Curr. Opin. Chem. Biol. 4, 324 (2000)
A. Dömling, Curr. Opin. Chem. Biol. 6, 306 (2002)
R. Vijayaraghavan, D.R. MacFarlane, Aust. J. Chem. 57, 129 (2004)
Y. Chauvin, L. Mussmann, H. Olivier, Angew. Chem., Int. Ed. 34, 2698 (1996)
M.A. Klingshirn, R.D. Rogers, K.H. Shaughnessy, J. Organomet. Chem. 690, 3620 (2005)
M. Johansson, A.A. Lindén, J.-E. Bäckvall, J. Organomet. Chem. 690, 3614 (2005)
A. Serbanovic, L.C. Branco, M. Nunes da Ponte, C.A.M. Afonso, J. Organomet. Chem. 690, 3600 (2005)
D. Zhao, Y. Liao, Z. Zhang, Clean-Soil. Air, Water. 35, 42 (2007)
O.G. da Silva, E.C.F. da Silva, M.G. Fonseca, L.N. Arakaki, C. Airoldi, J. Colloid Interface Sci. 302, 485 (2006)
K. Ghandi, Green Sustain. Chem. 4, 2160 (2014)
A. Akbari, N. Arsalani, M. Amini, E. Jabbari, J. Mol. Catal. A Chem. 414, 47 (2016)
A.E. Aksoylu, M. Madalena, A. Freitas, M.F.R. Pereira, J.L. Figueiredo, Carbon 39, 175 (2001)
Z. Yu, X. Min, F. Li, Q. Chen, D. Yin, Y. Peng, Mater. Technol. 33, 612 (2018)
M.-F. Huang, J.-G. Yu, X.-F. Ma, Polymer 45, 7017 (2004)
K. Molvinger, F. Quignard, D. Brunel, M. Boissière, J.-M. Devoisselle, Chem. Mater. 16, 3367 (2004)
S. Rogalsky, J.-F. Bardeau, S. Makhno, N. Babkina, O. Tarasyuk, T. Cherniavska, I. Orlovska, N. Kozyrovska, O. Brovko, Polymer 142, 183 (2018)
B.B.F. Mirjalili, F. Aref, Res. Chem. Intermed. 44, 4519 (2018)
R. Shojaei, M. Zahedifar, P. Mohammadi, K. Saidi, H. Sheibani, J. Mol. Struct. 1178, 401 (2019)
M. Zahedifar, A. Es-haghi, R. Zhiani, S.M. Sadeghzadeh, RSC Adv. 9, 6494 (2019)
M. Zahedifar, M. Shirani, A. Akbari, N. Seyedi, Cellulose 26, 6797 (2019)
M. Zahedifar, R. Zhiani, S.M. Sadeghzadeh, F. Shamsa, New J. Chem. 43, 4489 (2019)
R. Zhiani, S.M. Saadati, M. Zahedifar, S.M. Sadeghzadeh, Catal. Lett. 148, 2487 (2018)
A.D. Becke, Phys. Rev. A. 38, 3098 (1988)
P.J. Stephens, F.J. Devlin, C.F. Chabalowski, M.J. Frisch, J. Phys. Chem. 98, 11623 (1994)
R.A. Gaussian 09, G.W.T. M. J. Frisch, H. B. Schlegel, G. E. Scuseria, J.R.C. M. A. Robb, G. Scalmani, V. Barone, B. Mennucci, H.N. G. A. Petersson, M. Caricato, X. Li, H. P. Hratchian, J.B. A. F. Izmaylov, G. Zheng, J. L. Sonnenberg, M. Hada, K.T. M. Ehara, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, O.K. Y. Honda, H. Nakai, T. Vreven, J. A. Montgomery, Jr., F.O. J. E. Peralta, M. Bearpark, J. J. Heyd, E. Brothers, V.N.S. K. N. Kudin, R. Kobayashi, J. Normand, A.R. K. Raghavachari, J. C. Burant, S. S. Iyengar, J. Tomasi, N.R. M. Cossi, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, C.A. V. Bakken, J. Jaramillo, R. Gomperts, R. E. Stratmann, A.J.A. O. Yazyev, R. Cammi, C. Pomelli, J. W. Ochterski, K.M. R. L. Martin, V. G. Zakrzewski, G. A. Voth, J.J.D. P. Salvador, S. Dapprich, A. D. Daniels, J.B.F. O. Farkas, J. V. Ortiz, J. Cioslowski, G. and D. J. Fox, Inc., Wallingford CT, 2009
D.W. Smith, O.W. Day, J. Chem. Phys. 62, 113 (1975)
A. Bhowmick, D. Weatherman, P.P. Kundu, A.G. Sykes, Adv. Polym. Tech. 37, 235 (2016)
W. Lan, C.-F. Liu, F.-X. Yue, R.-C. Sun, J.F. Kennedy, Carbohydr. Polym. 86, 672 (2011)
X. Wang, P. Hu, F. Xue, Y. Wei, Carbohydr. Polym. 114, 476 (2014)
M. Zahedifar, P. Mohammadi, H. Sheibani, Lett. Org. Chem. 14, 315 (2017)
M. Zahedifar, R. Shojaei, H. Sheibani, Res. Chem. Intermed. 44, 873(2018)
S.J. Basha, S.P.V. Chamundeeswari, S. Muthu, B.R. Raajaraman, J. Mol. Liq. 296, 111787 (2019)
G. Singh, J. Sindhu, V. Kumar, V. Sharma, S.K. Sharma, S.K. Mehta, M.H. Mahnashi, A. Umar, R. Kataria, J. Mol. Liq. 296, 111814 (2019)
M. Zahedifar, H. Sheibani, V. Saheb, Synlett 29, 1836 (2018)
M. Zahedifar, R. Razavi, H. Sheibani, J. Mol. Struct. 1125, 730 (2016)
Acknowledgements
The authors express appreciation to the University of Jiroft Faculty Research Committee and Jiroft University of Medical Science for supporting this investigation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zahedifar, M., Pouramiri, B. & Razavi, R. Triethanolamine lactate-supported nanomagnetic cellulose: a green and efficient catalyst for the synthesis of pyrazolo[3,4-b]quinolines and theoretical study. Res Chem Intermed 46, 2749–2765 (2020). https://doi.org/10.1007/s11164-020-04117-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04117-8